Oct 29 2013
A UNIST undergrad, Minju Park, and her research team found a new way to synthesize highly efficient electrocatalysts based on heteroatom-doped graphene nanosheets.
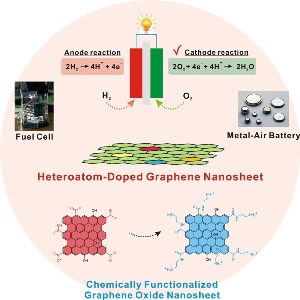 Overall Scheme for doped graphene oxide
Overall Scheme for doped graphene oxide
A Korean research team from Ulsan National Institute of Science and Technology (UNIST), S. Korea, developed a high performance and stable metal-free electrocatalyst for ORR and the research work was published in a science journal, Nanoscale by the Royal Society of Chemistry (RSC). (Title: “Covalent Functionalization Based Heteroatom Doped Graphene Nanosheet as a Metal-Free Electrocatalysts for Oxygen Reduction Reaction”)
Limited availability of fossil fuel and increasing energy demands have stimulated intense research on energy conversion and storage systems. Fuel cells have received considerable attention among the many choices of energy storage systems, owing to their remarkable potential energy density and environmental issues.
Electrocatalysts for oxygen reduction are critical components that may dramatically enhance the performance of fuel cells, which are perceived to be the power for future electric vehicles. For more economical fuel cells, engineers need fast and efficient electrocatalysts which split hydrogen gas to make electricity.
The UNIST research team led by Prof. Byeong-Su Kim from the Interdisciplinary School of Green Energy, UNIST, presented a unique design and characterization of new heteroatom-doped graphene nanosheets prepared through the covalent functionalization of various small organic molecules with a subsequent thermal treatment. This work was proposed and carried out by undergraduate student Minju Park from the Interdisciplinary School of Green Energy, UNIST.
There are many available methods to prepare nitrogen-doped (N-doped) graphene. These approaches successfully introduce nitrogen atoms within the graphene framework. However, many of them require toxic gas precursors, and are unable to control the degree of doping and type of nitrogen functionality.
Herein the UNIST Research team presented a simple approach for chemical functionalization toward heteroatom-dope graphene nanosheets with small organic molecules for use as electrocatalysts for the oxygen reduction reaction.
Here is how the material has been prepared:
Graphite oxide powder was prepared from graphite powder with oxidation and exfoliated to give a brown dispersion of graphene oxide (GO) under ultra sonication. Graphene oxide nanosheets have various functional groups on the edge such as carboxylic (-COOH), hydroxyl (-OH),and epoxy (-C-O-C).
When the GO suspension reacted with amines in the presence of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), a water soluble carbodiimide was usually obtained as the hyfrochloride, carboxylic group in GO reacted with amine and formed an amide group. The research team defined it as ‘NGOn’, which was chemically functionalized graphene oxide. NGOn suspensions were annealed at 800 ℃ for 1h under an argon atmosphere with tube furnace, and nitrogen was doped into the graphene oxide nanosheets with removing oxygen named ‘NRGOn’.
Further the UNIST research team demonstrated how the electrochemical performance can be improved by varying the degree and configurations of the nitrogen dopant. Further, they extended the approach toward the introduction of other heteroatoms, such as boron and sulfur, into the graphene nanosheet.
“Nitrogen-doped graphene nanosheets showed superior stability compared to commercial Pt/C catalysts. This approach has also been successfully extended to other heteroatoms such as boron and sulfur on the graphene nanosheets,” said Minju Park.
“We envision this study will offer opportunities and insights for further development of hybrid electrocatalysts,” said Prof. Kim, presenting future research possibilities.
This research work was supported by the National Research Foundation of Korea (NRF) grant.