Feb 12 2014
To improve their chances of success, in vitro fertilization (IVF) clinics need to assess the viability of the sperm they use. Now doctors may soon have a new technique to help them sort the good sperm cells from the less viable ones: a tracking system, developed by a team of researchers from four European institutions, that takes 3-D movies of living sperm.
In addition to showing the sperm's movement and behavior in real time, the novel method simultaneously provides detailed 3-D imaging of the sperm's form and structure to detect potential infertility-causing anomalies, such as the “bent tail” that prevents the cells from swimming straight.
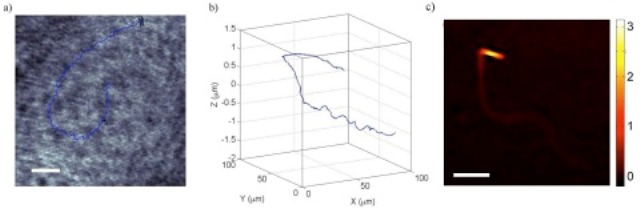 The track of a single sperm cell with a bent tail, in two dimensions (left) and three dimensions (right). Credit: Biomedical Optics Express.
The track of a single sperm cell with a bent tail, in two dimensions (left) and three dimensions (right). Credit: Biomedical Optics Express.
The researchers say this is the first technique for collecting data on sperm cell motility—a key predictor of IVF success—in three dimensions and over time. They describe their method in a paper published today in The Optical Society’s (OSA) open-access journal Biomedical Optics Express.
Currently, sperm concentration and mobility in semen are assessed either by subjective visual evaluation or a process known as computer-assisted sperm analysis (CASA). While the latter provides more detail and fewer errors than the former, CASA still only allows tracking and imaging in two dimensions. In their new technique, the team of researchers from Italy and Belgium combined microscopy and holography—the creation of 3-D images—to visualize live sperm in not only two dimensions (the x and y positions) but according to their depth (z position) as well.
And, “by acquiring a video of the moving sperm in 3-D, we add a fourth dimension – time,” said lead author Giuseppe Di Caprio of the Institute for Microelectronics and Microsystems of the National Research Council (NRC) in Naples, Italy, and Harvard University in Cambridge, Mass.
To achieve their new tracking system, the researchers first separated laser light into two beams. They transmitted one beam through a dish containing live, swimming sperm cells and then recombined it, after magnification through a microscope, with the second beam.
“The superimposed beams generate an interference pattern that we can record on camera,” Di Caprio explained. “The resulting image is a hologram containing information relative to the morphologies of the sperm and their positioning in three-dimensional space. Viewing a progressive series of these holograms in a real-time video, we can observe how the sperm move and determine if that movement is affected by any abnormalities in their shape and structure.”
Di Caprio says that the 3-D imaging technique, known formally as digital holographic microscopy (DHM), yields morphology and motility data on sperm consistent with that found in previous studies, but with the unprecedented bonus of seeing cause and effect relationships between the two.
“For example,” Di Caprio said, “we found that most of the sperm cells we observed swim along in one plane as expected. However, with the more detailed analysis provided by DHM, we also were able to show that this ‘in-plane’ movement –which we believe is linked to higher potential for fertility—does not occur when there are morphological anomalies such as sperm with misshapen heads or ‘bent tails.’”
Now that the efficiency of sperm tracking via DHM has been demonstrated, Di Caprio says that the international research team will next attempt to exploit its capabilities for defining the best-quality sperm for IVF.
“In one future experiment, we want to study sperm cells with vacuoles—enclosed compartments filled with water plus organic and inorganic molecules—that rest on the cell surface,” he said. Using DHM to assess the affected sperms’ motility, the team can determine if having vacuoles results in reduced fertility.
Di Caprio says that the long-term goal of such experiments and others using the new tracking system is to use the information gathered to develop a microchip-scale method for sorting good sperm cells from ones that are less viable.
The other institutions involved in this research are the National Institute of Optics of the NRC and the Center for Assisted Fertilization, both in Naples, and the Free University of Brussels in Belgium.
Paper: “4D tracking of clinical seminal samples for quantitative characterization of motility parameters,” Di Caprio, G. et al., Biomedical Optics Express, Vol. 5, Issue 3, pp. 690-700 (2014).