Apr 21 2014
Scientists at Rice University have created a nanoscale detector that checks for and reports on the presence of hydrogen sulfide in crude oil and natural gas while they’re still in the ground.
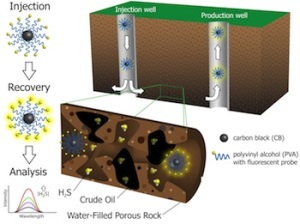 Fluorescent nanoreporters created at Rice University can tell oil producers how "sour" a reservoir is based on its hydrogen sulfide content. Illustration by Chih-Chau Hwang.
Fluorescent nanoreporters created at Rice University can tell oil producers how "sour" a reservoir is based on its hydrogen sulfide content. Illustration by Chih-Chau Hwang.
The nanoreporter is based on nanometer-sized carbon material developed by a consortium of Rice labs led by chemist James Tour and is the subject of a new paper published this month in the American Chemical Society journal ACS Applied Materials and Interfaces.
Limited exposure to hydrogen sulfide causes sore throats, shortness of breath and dizziness, according to the researchers. The human nose quickly becomes desensitized to hydrogen sulfide, leading to an inability to detect higher concentrations. That can be fatal, they said.
On the flip side, hydrogen sulfide is also a biologically important signaling molecule in processes that include pain and inflammation. Tour said chemists have synthesized fluorescent probes to detect it in the body. The Rice team capitalized on that work by using the probes to create downhole detectors for oil fields.
Crude oil and natural gas inherently contain hydrogen sulfide, which gives off a “rotten egg” smell. Even a 1 percent trace of sulfur turns oil into what’s known as “sour crude,” which is toxic and corrodes pipelines and transportation vessels, Tour said. The extra steps required to turn the sour into “sweet” crude are costly.
“So it’s important to know the content of what you’re pumping out of the ground, and the earlier the better,” Tour said.
Led by Rice professors Tour, Michael Wong and Mason Tomson and researcher Amy Kan, the university has pioneered efforts to gather information from oil fields through the use of nanoreporters. The nanoreporters were designed to detect and report on the presence and amount of oil in a well that might otherwise be hard to assess.
Now the same team, joined by chemist Angel Martí, is employing thermally stable, soluble, highly mobile, carbon black-based nanoreporters modified to look for hydrogen sulfide and report results immediately upon their return to the surface.
When exposed to hydrogen sulfide, the nanoparticles’ fluorescent properties immediately change. When pumped out of a production well, the particles can be analyzed with a spectrometer to determine the level of contamination.
“This paper is a big step because we’re making our nanoreporters detect something that’s not oil,” Wong said, suggesting the possibility that nanoparticles may someday be able to capture sulfur compounds before they can be pumped to the surface. “Even if that’s not cost-effective, just having information about the sulfur content may be enough to tell a company, ‘Let’s cap this well and move on to a cleaner site.’”
Modifying the particles with common polyvinyl alcohol (PVA) was the key to making the nanoreporters stable in temperatures as high as 100 degrees Celsius (212 degrees Fahrenheit). Testing in beds of sandstone or with actual Kuwaiti dolomite, to mimic oilfield environments, helped the team perfect the size and formula for nanoreporters that are most likely to survive a trip through the depths and return with data.
“We found the longer the PVA polymer chains, the more stable the nanoparticles were in the high temperatures they’re subjected to,” said Rice graduate student Chih-Chau Hwang, co-lead author of the paper with fellow graduate student Gedeng Ruan.
“The method of detection is so sensitive that large amounts of nanoreporters need not be pumped downhole,” Tour said. “This is enormously important for workers in the field to know for aspects of safety, lifetime of equipment and value of the afforded oil.”
Authors include graduate students Lu Wang, Errol Samuel, Changsheng Xiang, Zhiwei Peng and Kewei Huang; undergraduate student William Kasper; alumni Wei Lu and Zachary Schaefer; postdoctoral associate Haiyan Zheng; Kan, co-director of the Rice-based Brine Chemistry Consortium; Martí, an assistant professor of chemistry and bioengineering; Wong, a professor of chemical and biomolecular engineering and of chemistry; and Tomson, a professor of civil and environmental engineering. Tour is the T.T. and W.F. Chao Chair in Chemistry as well as a professor of mechanical engineering and materials science and of computer science.
The Advanced Energy Consortium supported the research.