May 22 2015
NIBIB-funded researchers have developed a highly sensitive and accurate imaging technique for non-invasive screening of lymph nodes for metastatic cancer. Current practice calls for invasive surgical biopsies to determine whether deadly metastatic cancer cells have invaded the lymph nodes.
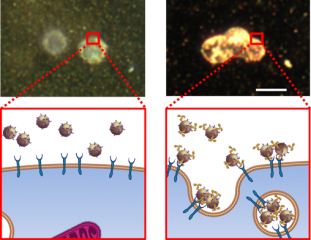 Upper left: A sPA signal cannot be obtained if the gold nanoparticles have no targeting antibody -- and thus cannot be captured by metastatic cells (cartoon bottom left). Upper right: A strong sPA signal is obtained from gold nanoparticles with a targeting antibody that are captured by the metastatic cells (cartoon bottom right).Credit:Adapted from Cancer Research, 2014, Oct 1;74(19):5397-408, Luke, et al. “Sentinel Lymph Node Biopsy Revisited: Ultrasound-Guided Photoacoustic Detection of Micrometastases Using Molecularly Targeted Plasmonic Nanosensors” with permission from AACR.
Upper left: A sPA signal cannot be obtained if the gold nanoparticles have no targeting antibody -- and thus cannot be captured by metastatic cells (cartoon bottom left). Upper right: A strong sPA signal is obtained from gold nanoparticles with a targeting antibody that are captured by the metastatic cells (cartoon bottom right).Credit:Adapted from Cancer Research, 2014, Oct 1;74(19):5397-408, Luke, et al. “Sentinel Lymph Node Biopsy Revisited: Ultrasound-Guided Photoacoustic Detection of Micrometastases Using Molecularly Targeted Plasmonic Nanosensors” with permission from AACR.
The new imaging technique – so far tested in mice – offers a rapid and effective tool to noninvasively identify very small numbers of these cells, known as micrometastases, thus detecting cancer’s spread at its earliest stages, which is critical for timely treatment.
The work, developed at the University of Texas at Austin and the University of Texas MD Anderson Cancer Center, is reported in the October issue of Cancer Research.1 The technique uses an imaging approach known as ultrasound-guided photoacoustics combined with nanosensors designed to target and identify metastatic cells in lymph nodes.
Richard Conroy, Ph.D., Director of the NIBIB Program in Molecular Imaging elaborates on the technology’s potential: "This work is an excellent example of the development of a cutting edge technology that works very well in an experimental system but also has great potential to change the way we monitor and diagnose cancer metastasis. Identifying the accumulation of cells early in the process with some molecular characterization offers the opportunity for more targeted and effective treatment and fewer side effects.”
More than 90% of cancer deaths can be attributed to metastases either directly or indirectly. In current clinical practice, an invasive surgical procedure called sentinel lymph node (SLN) biopsy is used to identify the regional spread of tumor. This procedure results in adverse effects including swelling, pain, numbness and risk of infection in hundreds of thousands of cancer patients per year.
In an effort to improve on accuracy and safety of lymph node biopsies, a number of noninvasive imaging modalities have been tested in animals and patients. Imaging techniques, including positron emission tomography (PET) and magnetic resonance imaging (MRI) have shown some potential, but currently lack the specificity and sensitivity to replace invasive lymph node biopsy. Knowing the shortcomings of previous attempts to use noninvasive imaging techniques, the UT research group developed a technology that is noninvasive and may have better sensitivity, accuracy and specificity than surgical biopsy.
The improvement in sensitivity and accuracy comes from the smart imaging probe that interacts with the metastatic cells. The group built a molecularly activated plasmonic nanosensor (MAPS) for this task. The MAPS components include a gold nanoparticle, which is the part of the nanosensor that is seen by the imaging system. The MAPS nanosensor also contains an antibody to the epidermal growth factor receptor (EGFR). This antibody was chosen because EGFR has been shown to be abnormally highly expressed on the surface of many cancer cells including lung, oral cavity, and cervix. With these two components the MAPS can find the metastatic cell using the antibody that binds to the EGFR receptor and can be seen using photoacoustic imaging systems that detect the gold nanoparticle, but, only when the MAPS interact with a cancer cell.
To detect the gold nanoparticles bound to metastatic cells in the lymph nodes, the researchers developed an ultrasound-guided spectroscopic photoacoustic (sPA) imaging system. The technology provides the high contrast and sensitivity of optical imaging with the ability of ultrasound to provide clear resolution even in tissues deep inside the body.
The researchers tested the system in a mouse model of oral cancer. The mice were injected with the EGFR-targeted MAPS and were subsequently imaged using sPA. The results indicated that the MAPS bound specifically to the metastatic cells in the lymph nodes near the oral cavity tumor and were clearly visible with the sPA imaging system. The ability of the MAPS to bind only to the metastatic cells in the lymph nodes, and the ability of the sPA imaging system to clearly detect cancer cells labeled with MAPS were impressive and extremely encouraging, according to the researchers.
Overall, tumor-bearing mice injected with the EGFR-targeted MAPS showed a sensitivity of 100% and a specificity of 87% for detection of lymph node micrometastases as small as 50 micrometers, which corresponds to about 30 metastatic cells. The detection of such a small number of cells in the lymph node offers a system that has the ability to identify metastasis very early in the process, which would allow early treatment.
“This combination greatly improves sensitivity and specificity of detection of cancerous cells in lymph nodes as compared to any other imaging modality in use today,” says Konstantin Sokolov, Ph.D., of the University of Texas MD Anderson Cancer Center and one of the senior authors. “Our method has a great potential to provide dramatic improvement in the clinical staging, prognosis, and therapeutic planning for cancer patients with metastatic disease without the need for invasive surgical biopsy,” adds Stanislav Emelianov, another senior author.
Although these are early studies in mice, the researchers are enthusiastic about translating the technology for use in humans as well as expanding the use of the system. In addition to the potential to identify cancer metastasis, the ability of the system to non-invasively image very small clusters of cells with high sensitivity and specificity opens the possibility of using the system to identify abnormal cells early in the process in a range of cancers as well as other conditions, such as cardiovascular disease.
The authors anticipate that this system can be translated into use in the clinic following some alterations in the system to make it functional and safe in humans. Necessary changes include identifying ultrasound frequencies that can penetrate to depths that would be needed in humans. In addition, potential toxicity of gold nanoparticles will have to be addressed, primarily by testing smaller nanoparticles that are efficiently cleared from the system but still are large enough to provide an adequate imaging signal.