Sep 23 2015
If you're reading this story on a screen with a liquid crystal display, thank thin-film transistors. Thin-film transistors function like standard semiconductor transistors, but are deposited on top of a layer of glass. In LCD screens, this allows the transistors to be embedded directly in the screen, which improves image stability.
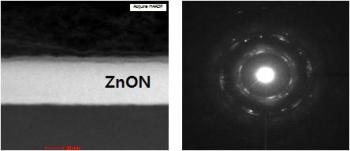 This is a cross-sectional high angle annular dark field scanning transmission electron microscopy (HAADF-STEM) image and nanobeam diffraction pattern of Ar plasma treated ZnON. Credit: E. Lee & S. Jeon/Samsung Advanced Institute of Technology & Korea University
This is a cross-sectional high angle annular dark field scanning transmission electron microscopy (HAADF-STEM) image and nanobeam diffraction pattern of Ar plasma treated ZnON. Credit: E. Lee & S. Jeon/Samsung Advanced Institute of Technology & Korea University
Researchers at Korea University and the Samsung Advanced Institute of Technology have now developed a new type of thin film transistor that's significantly faster than its predecessors -- an important step toward speeding up image display on devices like TVs and smartphone screens. The scientists made the transistor from zinc oxynitride, or ZnON, which they then plasma treated with argon gas.
Much of the thin-film research involving zinc oxide materials has focused on the addition of metal cations, such as indium with gallium, hafnium, zirconium and lanthanide. When used in semiconductors, these exhibit mobility values -- the speed at which electrons subjected to an electric field travel through a material -- from 5 to 20 cm2/voltseconds. While extreme electron mobilities have been recorded under ideal lab conditions -- graphene's vaunted mobility is around 200,000 cm2/voltseconds -- thin-film transistors speeds in commercial electronics largely fall within the single to double digit range.
"For future electronic devices, the mobility of the active oxide channel materials needs to be further increased above 100 centimeters squared per voltseconds," said Sanghun Jeon, an associate professor in the Department of Applied Physics and Department of Display and Semiconductor Physics at Korea University.
Jeon's group and their colleagues in the Analytical Engineering Group at the Samsung Advanced Institute of Technology built a ZnON transistor with electron mobilities about ten times greater than their previous thin-film transistors. They present their work this week in Applied Physics Letters, from AIP Publishing.
Active oxide semiconductors, such as ZnON, provide a variety of benefits, such as a low cost of production and a relatively low temperature of fabrication -- below 300° C -- which makes them suitable for display applications and makes it easy to integrate them with a variety of other inorganic and organic materials.
As a thin film, zinc oxynitride -- a glassy composite of ZnO, ZnOxNx and Zn3N2 -- exhibits extremely fast mobility rates due to its ability to deactivate oxygen vacancies, which are defects in transition metal oxide surfaces that occur due to inevitable bonding flaws in creating the compound. When the oxygen anion in ZnO is substituted with a nitrogen anion, the valence band edge, or fully occupied energy band edge, of ZnON is positioned above the previous location of the neutral oxygen vacancy in ZnO, effectively burying the vacancy below the valence band edge and preventing gaps in conductance.
The researchers constructed the zinc oxynitride thin films by sputter-depositing a mixture of N2, O2 and argon gases through a rotating holder onto a zinc target. To control the anion contents in the films, the O2 partial pressure in the chamber was varied during the deposition by adjusting its flow rate, while the nitrogen and argon rates were kept constant. This resulted in a glassy, composite film that was roughly 50 nanometers thick, but relatively unstable due to nitrogen's low reactivity with zinc relative to atmospheric oxygen.
To address this, the researchers adopted the argon plasma process, in which a film is bombarded with the high-energy plasma form of the noble gas. This caused cascades of atomic and ionic collisions and energy dissipation inside the material, which reordered the bonds between the zinc, oxygen and nitrogen atoms into a chemically uniform nanocrystalline structure in an amorphous matrix. This had the effect of improving the material's stability under radiation and electrical stress.
Additionally, when compared to an untreated film after both were exposed to atmospheric conditions for 30 days, the researchers found that the treated film exhibited no evidence of nitrogen loss through diffusion.
When subjected to conductance tests, the channel mobility, or semiconductor speed, of the nanocrystalline thin film transistor was found to be 138 cm2/vs -- an order of magnitude higher than that of the group's previous indium-gallium-zinc-oxide film.
"We believe that zinc oxynitride, tailored by reactive sputtering and plasma processes, will constitute another significant breakthrough in the field of thin film electronics," Jeon said.
Future work for Jeon and his colleagues includes incorporating other metal cations in their metal oxynitrides, as well as exploring the effects of plasma and vacuum UV exposure on the films.