Apr 6 2016
Researchers from the Lawrence Livermore National Laboratory (LLNL) have demonstrated for the first time that carbon nanotubes, whose diameter measure almost eight-tenths of a nanometer, are capable of transporting protons in a more rapid manner than bulk water, by an order of magnitude.
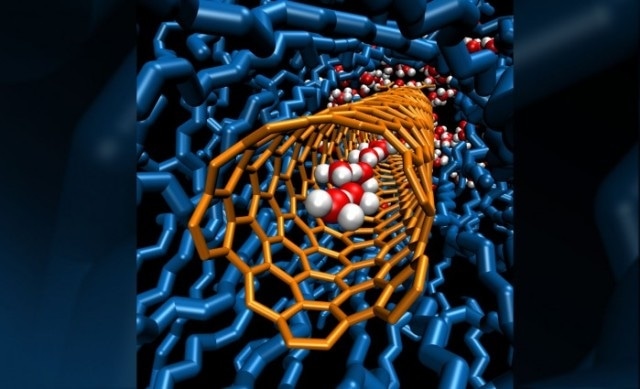 A single chain of water molecules lines the cavity inside a carbon nanotube porin, which is embedded in a lipid bilayer. Image by: Y. Zhang and Alex Noy/LLNL.
A single chain of water molecules lines the cavity inside a carbon nanotube porin, which is embedded in a lipid bilayer. Image by: Y. Zhang and Alex Noy/LLNL.
A 200-year old mechanism of proton transport was analyzed as part of the research.
Generally, a nanometer refers to one billionth of a meter. In comparison to this, the diameter of a human hair measures 20,000 nanometers.
These nanotube pores that develop one-dimensional water wires exceed those of man-made proton conductors and biological channels, making carbon nanotubes to be the fastest known proton conductor. The research is featured in the latest online issue of Nature Nanotechnology.
Applications of the proton conductor include the upcoming area of proton bioelectronics (protonics), proton-based signaling in biological systems, and proton exchange membranes.
The cool thing about our results is that we found that when you squeeze water into the nanotube, protons move through that water even faster than through normal (bulk) water.
Aleksandr Noy, Biophysicist, LLNL
(Bulk water is similar to what you would find in a cup of water that is much bigger than the size of a single water molecule).
The concept that protons can be transported rapidly in solutions by jumping along hydrogen-bonded water molecule chains refers to the work of Theodore von Grotthuss, from around 200 years ago and continues to remain the basis of the scientific understanding of the transport of proton.
As part of the research work, LLNL researchers used carbon nanotube pores to assemble water molecules into accurate one-dimensional chains. The researchers then allowed the transport rates of protons to reach the optimum limits for the Grotthuss transport mechanism.
The possibility to achieve fast proton transport by changing the degree of water confinement is exciting. So far, the man-made proton conductors, such as polymer Nafion, use a different principle to enhance the proton transport. We have mimicked the way biological systems enhance the proton transport, took it to the extreme, and now our system realizes the ultimate limit of proton conductivity in a nanopore.
Aleksandr Noy, Biophysicist, LLNL
When compared to man-made materials, the narrow hydrophobic inner pores found in carbon nanotubes (CNT) could prove suitable to deliver weak interactions and the confinement level with water molecules that help to form one-dimensional hydrogen-bonded water chains that improve the transport of protons.
Initial molecular dynamic simulations highlighted that water present in 0.8 nm diameter carbon nanotubes would form such water wires. These simulations also predicted that these channels would be capable of displaying proton transport rates that are more rapid than the transport rates of bulk water.
Ramya Tunuguntla, an LLNL postdoctoral researcher and the first author on the paper, stated that in spite of the vital efforts carried out in studies related to carbon nanotube transport, there has been a difficulty in validating these predictions, mainly due to the issues in developing CNT pores of sub-1 nm diameter.
Nevertheless, the Lawrence Livermore team together with a group of colleagues from UC Berkeley and the Lawrence Berkeley National Lab succeeded in developing a simple experimental system to analyze the transport process in ultra-narrow CNT pores. The team had previously developed a technology called carbon nanotube porins (CNTPs) at LLNL. This technology uses carbon nanotubes implanted in the lipid membrane to mirror the working of the biological ion channel. The major success here is the development of nanotube porins comprising a diameter of less than 1 nm, which allows researchers - for the very first time - to obtain an accurate one-dimensional water confinement.
The research was financially supported by the Department of Energy’s Office of Basic Energy Sciences. Other Berkeley and Livermore researchers who were part of this research include Allison Belliveau, Kyunghoon Kim, and Frances Allen.