May 24 2016
Researchers at Rice University have developed a graphene-based de-icer to provide dual purpose. The novel material continues to melt ice from wings and wires when it becomes too cold. However, if the air is above 7°F, ice would not form at all.
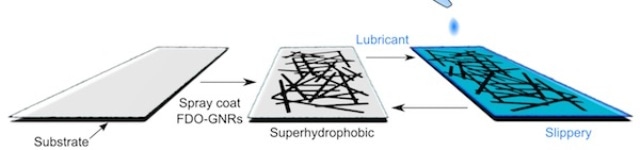 Rice scientists have modified their graphene-based de-icer to resist the formation of ice well below the freezing point and added superhydrophobic capabilities. The robust film is intended for use in extreme environments as well as on aircraft, power lines and ships. Illustration courtesy of the Tour Group
Rice scientists have modified their graphene-based de-icer to resist the formation of ice well below the freezing point and added superhydrophobic capabilities. The robust film is intended for use in extreme environments as well as on aircraft, power lines and ships. Illustration courtesy of the Tour Group
Chemist James Tour’s Rice lab provided its de-icer super hydrophobic (water-repelling) abilities that inertly avoid water from freezing above 7°. The hard film formed when the de-icer is sprayed onto a surface, and it consists of atom-thin graphene nanoribbons which are conductive, so the material could also be heated by electricity to melt snow and ice in cooler conditions.
According to the researchers, this material could be spray-coated, making it suitable for huge applications such as power lines, aircraft, ships, and radar domes. This work was published in the American Chemical Society journal ACS Applied Materials and Interfaces.
We’ve learned to make an ice-resistant material for milder conditions in which heating isn’t even necessary, but having the option is useful. What we now have is a very thin, robust coating that can keep large areas free of ice and snow in a wide range of conditions.
James Tour, Chemist, Rice University
Tour, lead authors Tuo Wang, a Rice graduate student, and Yonghao Zheng, a Rice postdoctoral researcher, and their co-workers analyzed the film on plastic and glass.
Materials are superhydrophobic, provided they contain a water-contact angle greater than 150°. This term refers to the angle at which the water surface comes into contact with the surface of the material. In other words, the larger the beading, the greater the angle. A 0° angle is essentially a puddle, whereas a maximum angle of 180°describes a sphere slightly touching the surface.
The Rice films use graphene nanoribbons, altered with a fluorine compound to improve their hydrophobicity. The scientists discovered that nanoribbons altered with longer perfluorinated chains gave rise to films with a greater contact angle, implying that the films are adjustable for specific conditions. Tou suggested that warming the test surfaces to room temperature, followed by re-cooling had no effect on the properties of the film.
The scientists found that below 7°, water could condense within the pores of the structure, making the surface lose both its ice-phobic and superhydrophobic properties. At that time, applying a minimum of 12 V of electricity heated them adequately which enabled it to retain its repellant properties. The film was brought back to room temperature when applied with 40 V of electricity, even though the ambient temperature was 25° below zero. Ice that was permitted to form at that temperature melted following 90 seconds of resistive heating.
The scientists discovered that while being effective, the de-icing mode did not eliminate water totally, as some droplets remained entrapped within the pores among the joined nanoribbon bundles. Addition of a low melting point (-61°F) lubricant to the film rendered the surface slippery, saved energy, and sped de-icing.
The paper’s co-authors are Abdul-Rahman Raji, a Rice alumnus and currently a postdoctoral researcher at the University of Cambridge; and Yilun Li and William Sikkema, Rice graduate students. Tour is a professor of computer science and of materials science and nanoengineering and also T.T. and W.F. Chao Chair in Chemistry.
This project was funded by Air Force Office of Scientific Research.