Aug 10 2016
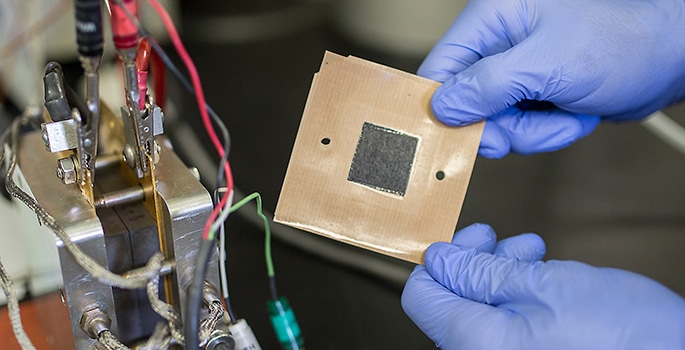 Nanofiber mat electrode (John Russell / Vanderbilt)
Nanofiber mat electrode (John Russell / Vanderbilt)
Honda and Toyota are simultaneously launching fuel cell cars in the U.S. market. Researchers from Vanderbilt University, Nissan North America and Georgia Institute of Technology have worked together to develop a new technology that provides more 'oomph' to fuel cells.
The project, which aims to improve the durability and performance of fuel cells and the hydrogen storage technologies, is part of a $13 million Department of Energy program.
The $4.5 million alliance is based on a new nanofiber mat technology created by Peter Pintauro, the H. Eugene McBrayer Professor of Chemical Engineering at Vanderbilt. This new technology replaces the standard electrodes used in fuel cells.
In comparison to the standard catalyst layers, the nanofiber electrodes are less expensive, have more durability and can also increase the power output of fuel cells by 30%.
Vanderbilt has patented the new technology, and license has been obtained by Merck KGaA in Germany, who is working with leading auto manufacturers focusing on applying this new technology to the next generation of automotive fuel cells.
Fuel cells were invented in 1839, but they were first seen in the 'real world' from the 1960’s when NASA used these cells to power the Apollo spacecraft. Fuel cells, just like a gasoline engine, run on fuel and air but they produce electricity like a battery.
Hydrogen passes through only one side of the device in hydrogen/air fuel cells. The other side is pumped with air. The hydrogen is oxidized into protons at the anode. The protons travel to the cathode where channeling of the air takes place, decreasing the oxygen content to form water.
Specific catalysts in the cathode and anode enable these reactions to take place spontaneously, leading to the production of electricity in the process. Fuel cells transform fuel to electricity with 40% to 60% efficiency. These cells are extremely quiet as they do not contain moving parts. Fuel cells are also environmentally friendly as water is the only waste product.
Porous Polymer Fiber Mats Replace Solid Electrodes
Thin sheets of catalyst particles blended with a polymer binder are used by the standard fuel cells for the electrodes. In general, the catalyst is platinum on carbon powder. These solid sheets are replaced by the Vanderbilt approach with mats produced from a knot of polymer fibers that are each a fraction of the thickness of a strand of human hair developed by a method known as electrospinning.
Catalyst particles are fixed to the fibers. The extremely small diameter of the fibers refers to the existence of a larger surface area of catalyst available for reactions caused by hydrogen and oxygen gas during fuel cell operation.
The pores existing between fibers in the mat electrode also play a major role in removing the waste water. Increase fuel cell power with cost effective platinum is obtained because of the unique structure of the fiber electrode.
Octagonal Catalyst Particles More Effective
In the meantime, Yuounan Xia, biomedical engineering professor at Georgia Tech, invented a method for efficiently monitoring the shape of the nanoparticle catalyst for fuel cells. He specifically developed platinum-nickel nanoparticles with a normal octagonal shape.
These particles should theoretically be a lot effective than the commercially available platinum black powder, which is currently used as the oxygen reduction catalyst in air fuel/hydrogen cells.
The combination of the Georgia Tech catalyst with Vanderbilt’s nanofiber electrode technology could be a game-changer for the development and commercialization of automotive fuel cells.
Peter Pintauro, Professor, Vanderbilt University
Pintauro’s team, as part of the DOE project, will develop nanofiber mat electrodes comprising Xia’s nanoparticle catalysts. These electrodes will then be delivered to Nissan Technical Center North America where a research team headed by Nilesh Dale, the manager of fuel cell research, will examine their performance in automotive operating conditions.
The researchers will also be collaborating with scientists at the national labs in order to enhance the scientific understanding of why fuel cells function better with nanofiber mat electrodes. These national labs include Oak Ridge National Laboratory, Lawrence Berkeley National Laboratory, the National Renewable Energy Laboratory and Los Alamos National Laboratory.
Right now, much of the research work on fuel cell electrodes is very Edisonian. It is mainly trial and error experiments. We don’t know what will happen when we change the composition or structure of the electrodes in hydrogen/air fuel cells. With a better understanding of the interdependence of composition and nanostructure for fiber electrodes, we could accelerate the pace of our research, which would help us to achieve the cost and performance targets needed for automotive fuel cell commercialization.
Peter Pintauro, Professor, Vanderbilt University