Apr 12 2017
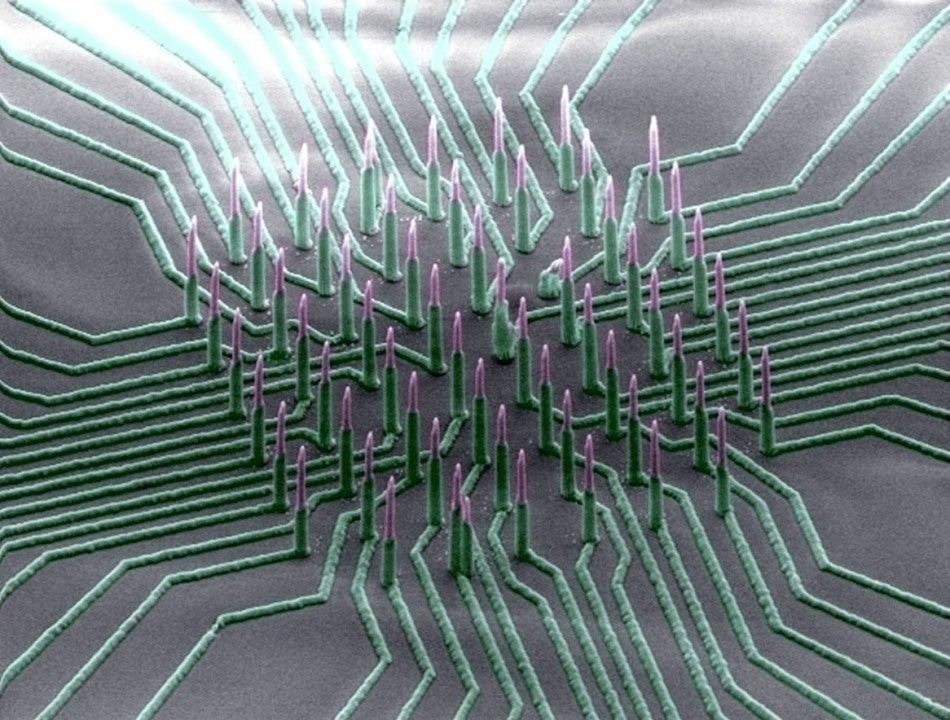 Colorized SEM image of the nanowire array. (Image courtesy of Integrated Electronics and Biointerfaces Laboratory, UC San Diego)
Colorized SEM image of the nanowire array. (Image courtesy of Integrated Electronics and Biointerfaces Laboratory, UC San Diego)
Nanowires capable of recording the electrical activity of neurons in fine detail have been developed by a research team led by engineers at the University of California San Diego. This new nanowire technology could be a futuristic platform to screen drugs for neurological diseases and could enable scientists to properly understand how single cells communicate in large neuronal networks.
We’re developing tools that will allow us to dig deeper into the science of how the brain works.
Professor Shadi Dayeh, Jacobs School of Engineering, UC San Diego
“We envision that this nanowire technology could be used on stem-cell-derived brain models to identify the most effective drugs for neurological diseases,” said Anne Bang, director of cell biology at the Conrad Prebys Center for Chemical Genomics at the Sanford Burnham Medical Research Institute.
The project was a joint effort between the Dayeh and Bang labs, neurobiologists at UC San Diego, and researchers at Nanyang Technological University in Singapore and Sandia National Laboratories. The details of the research project have been published on April 10 in Nano Letters.
Scientists can discover details about a neuron’s health, response, and activity to drugs by measuring ion channel currents and alterations in its intracellular potential, which is because of the difference in ion concentration between the outer and inner sides of the cell. The advanced measurement method is sensitive to minor potential changes and delivers readings with high signal-to-noise ratios. However, this technique is damaging — it can break the cell membrane and ultimately kill the cell. It is also restricted to examining just one cell at a time, making it unfeasible for investigating large networks of neurons, which are how they are naturally set in the body.
Existing high sensitivity measurement techniques are not scalable to 2D and 3D tissue-like structures cultured in vitro. The development of a nanoscale technology that can measure rapid and minute potential changes in neuronal cellular networks could accelerate drug development for diseases of the central and peripheral nervous systems.
Professor Shadi Dayeh, Jacobs School of Engineering, UC San Diego
The nanowire technology developed in Dayeh’s laboratory is non-destructive and can concurrently measure probable changes in numerous neurons — with the high sensitivity and resolution accomplished by the existing state of the art.
The device comprises of a collection of silicon nanowires tightly packed on a small chip patterned with nickel electrode leads that are coated with silica. The nanowires poke into cells without destroying them and are sufficiently sensitive to measure minor potential changes that are a fraction of or a few millivolts in magnitude. The research team used the nanowires to record the electrical activity of neurons that were taken from mice and derived from human induced pluripotent stem cells. These neurons endured and continued working for nearly six weeks while connected with the nanowire array in vitro.
Another ground-breaking feature of this technology is it can isolate the electrical signal measured by each distinct nanowire.
This is unusual in existing nanowire technologies, where several wires are electrically shorted together and you cannot differentiate the signal from every single wire.
Professor Shadi Dayeh, Jacobs School of Engineering, UC San Diego
To surpass this difficulty, the team invented a new wafer bonding method to fuse the silicon nanowires to the nickel electrodes. Their method involved a process called silicidation, where a reaction helps to bind two solids (silicon and another metal) together without melting both of the materials. This process prevents the nickel electrodes from liquidizing, dispersing, and shorting neighboring electrode leads.
Silicidation is typically used to build contacts to transistors, but this is the first time it is being used to perform patterned wafer bonding, Dayeh said. “And since this process is used in semiconductor device fabrication, we can integrate versions of these nanowires with CMOS electronics.” Dayeh’s laboratory holds a number of pending patent applications for this technology.
Dayeh explained that the technology requires additional optimization for brain-on-chip drug screening. His team is keen to extend the application of the technology to heart-on-chip drug screening for in vivo brain mapping and cardiac diseases, which is still more than a few years away due to substantial biological and technological challenges that the researchers will have to resolve. “Our ultimate goal is to translate this technology to a device that can be implanted in the brain.”