Jun 29 2017
By definition, dialysis is a process in which molecules are filtered out of a solution by getting diffused through a membrane into a comparatively dilute solution. Apart from hemodialysis in which waste is eliminated from blood, Researchers employ dialysis for removing residue from chemical solutions, purifying drugs and for isolating molecules for medical diagnosis, generally by making the materials to go through a porous membrane.
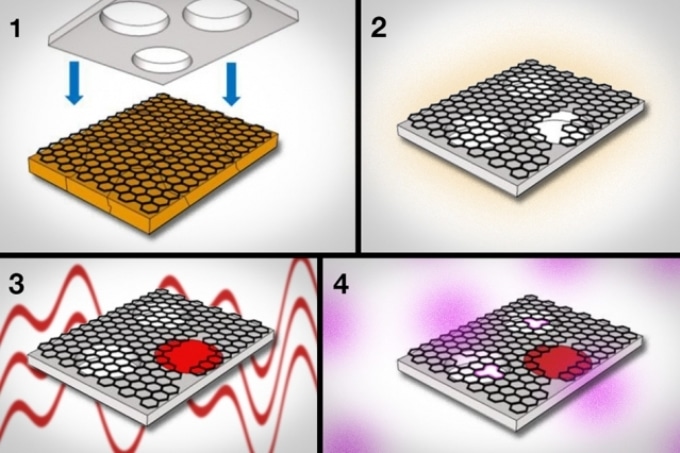 Caption: 1) Graphene, grown on copper foil, is pressed against a supporting sheet of polycarbonate. 2) The polycarbonate acts to peel the graphene from the copper. 3) Using interfacial polymerization, researchers seal large tears and defects in graphene. 4) Next, they use oxygen plasma to etch pores of specific sizes in graphene. CREDIT: Courtesy of the researchers (edited by MIT News).
Caption: 1) Graphene, grown on copper foil, is pressed against a supporting sheet of polycarbonate. 2) The polycarbonate acts to peel the graphene from the copper. 3) Using interfacial polymerization, researchers seal large tears and defects in graphene. 4) Next, they use oxygen plasma to etch pores of specific sizes in graphene. CREDIT: Courtesy of the researchers (edited by MIT News).
Prevalent commercial dialysis membranes are very slow in isolating molecules partially because of their structure ‒ they are comparatively thick, and the pores formed in these dense membranes have winding paths, rendering it tougher for target molecules to swiftly pass through.
At present, Engineers from MIT have synthesized an effective dialysis membrane using a graphene sheet, that is, a single carbon atom layer in which the atoms are connected end to end in hexagonal arrangement similar to chicken wire. The thickness of the graphene membrane, whose size is roughly equal to that of a fingernail, is less than 1 nm. The lowest thickness of the prevalent membranes is nearly 20 nm. The membrane developed by the MIT Researchers has the capacity to filter out even nanometer-scale molecules from aqueous solutions nearly 10 times quicker than the most modern membranes, where graphene is itself nearly 100 times quicker.
Although graphene has been chiefly investigated for use in the field of electronics, according to Piran Kidambi, a Postdoc in the Department of Mechanical Engineering at MIT, the discovery of the research team indicates that graphene has the ability to enhance membrane technology, specifically for lab-scale separation procedures and prospectively for hemodialysis.
Because graphene is so thin, diffusion across it will be extremely fast. A molecule doesn’t have to do this tedious job of going through all these tortuous pores in a thick membrane before exiting the other side. Moving graphene into this regime of biological separation is very exciting.
Piran Kidambi, a Postdoc in the Department of Mechanical Engineering, MIT
The study was reported in the journal Advanced Materials on 28th June 2017. The Lead Author of the study is Kidambi, and all the six Co-Authors of the study—including Rohit Karnik, Associate Professor of Mechanical Engineering, and Jing Kong, Associate Professor of Electrical Engineering—belong to MIT.
Plugging graphene
In order to synthesize the graphene membrane, the research team initially employed a regular method known as chemical vapor deposition, for growing graphene on copper foil. Subsequently, they cautiously etched away the copper and shifted the graphene onto a polycarbonate supporting sheet whose surface is distributed with pores of size adequately big to let in any molecules that passed through the graphene. The polycarbonate functions like a scaffold and prevents curling up of the ultrathin graphene.
The research team attempted to transform graphene into a molecularly selective sieve that allows only molecules of specific size to pass through. For achieving this, they made minute pores on the material by treating the structure with oxygen plasma, a procedure in which oxygen pumped into a plasma chamber etches away materials.
By tuning the oxygen plasma conditions, we can control the density and size of pores we make, in the areas where the graphene is pristine. What happens is, an oxygen radical comes to a carbon atom [in graphene] and rapidly reacts, and they both fly out as carbon dioxide.
Piran Kidambi, a Postdoc in the Department of Mechanical Engineering, MIT
What remains is a minute hole in the graphene, the place at which once there was a carbon atom. Kidambi and his collaborators discovered that the length of time oxygen plasma is in contact with graphene is directly proportional to the size and density of the pores. Shorter exposure times of around 45-60 seconds result in extremely small pores.
Desirable defects
The research team investigated numerous graphene membranes that had pores of different distributions and sizes, locating each membrane at the center of a diffusion chamber. The feed side of the chamber was filled with a solution comprising of different mixtures of molecules that have varied sizes, such as potassium chloride with a width of 0.66 nm, vitamin B12 with a width of 1-1.5 nm, and lysozyme, a protein contained in egg white, with a width of 4 nm. A dilute solution was filled in the other side of the chamber.
Then, the Researchers measured the molecular flow as the molecules diffused through each of the graphene membranes.
Membranes that had minute pores allowed potassium chloride to pass through but blocked larger molecules such as L-tryptophan with a width of just 0.2 nm. Membranes that had larger pores allowed comparatively larger molecules to pass through.
The Researchers performed almost identical experiments by using commercial dialysis membranes and discovered that when compared with prevalent membranes, graphene membranes had higher “permeance,” and filtered out the requisite molecules nearly 10 times quicker.
Kidambi noted that the pores etched in the polycarbonate support occupy just 10% of its surface area, thus restricting the amount of requisite molecules that eventually pass through both layers.
“Only 10 percent of the membrane’s area is accessible, but even with that 10 percent, we’re able to do better than state-of-the-art,” stated Kidambi.
To make the graphene membrane even better, the team plans to improve the polycarbonate support by etching more pores into the material to increase the mebrane's overall permeance. Furthermore, they are in the process of increasing the dimensions of the membrane, which at present are 1 cm2. The performance of the membrane can be further enhanced by fine tuning the oxygen plasma process to etch customized pores, which according to Kidambi will have largely differing outcomes for graphene in electronics applications.
What’s exciting is, what’s not great for the electronics field is actually perfect in this [membrane dialysis] field. In electronics, you want to minimize defects. Here you want to make defects of the right size. It goes to show the end use of the technology dictates what you want in the technology. That’s the key.
Piran Kidambi, a Postdoc in the Department of Mechanical Engineering, MIT
The U.S. Department of Energy and a Lindemann Trust Fellowship partially supported the study.