Feb 25 2018
Researchers at Weill Cornell Medicine have discovered a new cellular messenger which might assist in finding out the way cancer cells get assimilated into the intercellular delivery service of the human body to proliferate to new locations.
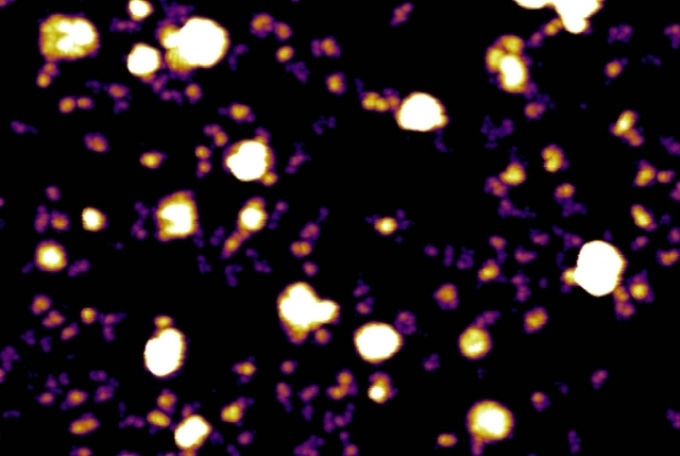 Exosomes (white ball-like structures) and exomeres (purple and yellow) secreted by melanoma tumor cells. This image was taken using a type of microscopy called Atomic force microscopy, which uses high-resolution imaging to visualize nanoparticles. (Image credit: Molecular Cytology Core Facility, Memorial Sloan Kettering Cancer Center.)
Exosomes (white ball-like structures) and exomeres (purple and yellow) secreted by melanoma tumor cells. This image was taken using a type of microscopy called Atomic force microscopy, which uses high-resolution imaging to visualize nanoparticles. (Image credit: Molecular Cytology Core Facility, Memorial Sloan Kettering Cancer Center.)
The researchers have reported in a paper published in Nature Cell Biology on February 19, 2018, that a more advanced method known as asymmetric flow field-flow fractionation (AF4) can effectively sort nano-sized particles, called exosomes, released by cancer cells and containing fats, DNA, proteins, and RNA. The technique enabled the researchers to isolate two different subtypes of exosomes and to find out a new nanoparticle, named exomeres.
“We found that exomeres are the most predominant particle secreted by cancer cells,” stated Dr. David Lyden, senior author of the study, who is the Stavros S. Niarchos Professor in Pediatric Cardiology and a scientist in the Sandra and Edward Meyer Cancer Center and the Gale and Ira Drukier Institute for Children’s Health at Weill Cornell Medicine. “They are smaller and structurally and functionally distinct from exosomes. Exomeres largely fuse with cells in the bone marrow and liver, where they can alter immune function and metabolism of drugs. The latter finding may explain why many cancer patients are unable to tolerate even small doses of chemotherapy due to toxicity.”
The diameter of exomeres is less than 50 nm as against small exosomes (Exo-S) with a diameter of 60-80 nm, and large exosomes (Exo-L) with a diameter of 90-120 nm.
“Exosomes and exomeres also have different biophysical characteristics, such as stiffness and electric charge, that likely affect their behavior in the body,” stated Dr. Haiying Zhang, lead author of the study, who is an assistant professor of cell and developmental biology in pediatrics at Weill Cornell Medicine. “The more rigid the particle, the easier it is likely taken up by cells, rendering exomeres, which are stiffer than exosomes, the more effective messengers of transferring tumor information to recipient cells.”
There is a distinction between exomeres and exosomes in terms of their influence on cancer. Exomeres transport metabolic enzymes to the liver, an organ important for disintegrating drugs into nontoxic forms. The discovery indicates that exomeres attack the liver and “reprogram” its metabolic function to induce the advancement of the tumor. Exomeres also transport blood-clotting factors to the liver, thereby hindering the normal function of liver in regulating clotting. Conversely, the research indicates that Exo-L might boost metastasis to lymph nodes, whereas Exo-S might assist in distant metastasis.
Cancer is truly a systemic disease that requires multi-organ involvement to progress. Our finding that tumor cells secrete these three distinct nanoparticles, that then target cells in different organs reflects this important aspect of the disease.
Dr. David Lyden, Senior Author
A patent application on the technology, reported in the Nature Cell Biology paper, has been filed by Cornell University. In the due course, scientists will investigate the way in which the various messengers develop, the molecules carried by each of them, and their functions at the target organ sites.
Understanding these characteristics may help scientists better understand how exomeres and exosomes help cancers grow and spread to other organs, as well as what role they may play in other diseases.
Dr. Haiying Zhang, Lead Author
Exosomes and exomeres can also be found in bodily fluids (for example, lymphatic fluid), enabling the development of biomarkers for early detection of cancer or similar pathological conditions.
“Based on our findings, the next phase will be to measure exosomes and exomeres in plasma samples to help predict organs that may be targeted for metastasis during tumor progression,” stated Dr. Lyden. “This will help us better understand the biology of cancer, guide therapeutic decisions and develop novel therapies.”
“Lastly, the technique we have pioneered will likely be a valuable tool for scientists and clinicians studying the biology of complex nanoparticle populations,” added Dr. Zhang, “and may aid in the development of diagnostic tests using them as biomarkers.”