Oct 16 2018
Nanodiamonds - the tiny crystalline carbon - have fascinating chemical and surface properties and hold promising applications in quantum computing, optoelectronics, and medicine. These materials measure hundreds of thousands of times smaller than a single grain of sand.
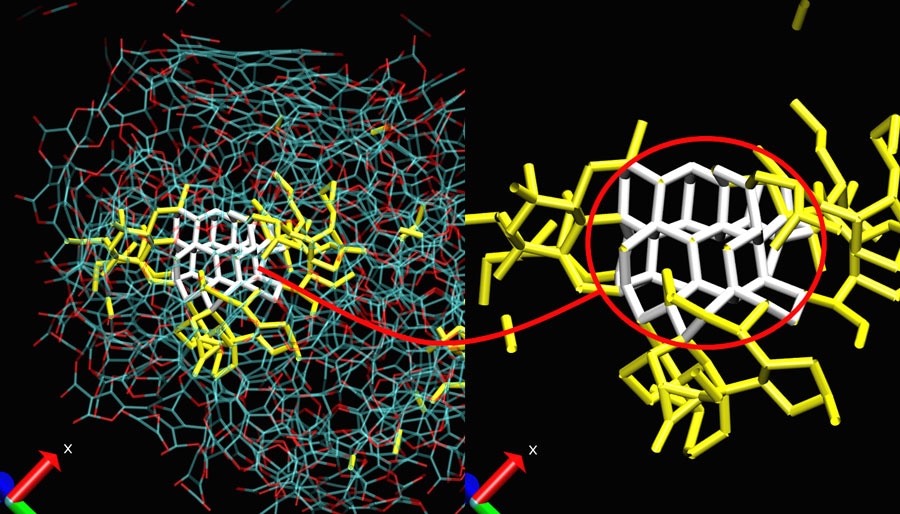 A carbonaceous nanoparticle (left) and its pure carbon core (right) Blue: carbon atoms; red: oxygen atoms; white: diamond seed; yellow: pure carbon network surrounding the diamond seed. (CREDIT: X. Bidault and N. Pineau)
A carbonaceous nanoparticle (left) and its pure carbon core (right) Blue: carbon atoms; red: oxygen atoms; white: diamond seed; yellow: pure carbon network surrounding the diamond seed. (CREDIT: X. Bidault and N. Pineau)
In order to counterfeit these nanoscopic gemstones, organic explosive molecules are exposed to intense detonations in a controlled environment.
However, these explosive forces do not make it easy to analyze the formation of nanodiamonds, even within the laboratory conditions.
In an effort to resolve this obstacle, two French researchers came up with a novel procedure, as well as a computer model, that is capable of simulating the highly unpredictable conditions of explosions on incredibly short time scales.
The researchers have reported their work in AIP Publishing’s The Journal of Chemical Physics.
Understanding the processes that form nanodiamonds is essential to control or even tune their properties, making them much better suited for specific purposes.”
Xavier Bidault, Co-author
A type of simulation called Reactive Molecular Dynamics was used by Bidault and his co-author Nicolas Pineau.
This simulation replicates the time evolution of systems that are complex and chemically reactive down to the atomic level.
The atomic-level interaction model is essential to really understand what’s happening. It gives us an intimate way to analyze, step-by-step, how carbon-rich compounds can form nanodiamonds in a high-pressure, high-temperature system.”
Nicolas Pineau
However, actual experimental analysis is not feasible because a detonation has extreme and fleetingly brief conditions.
As a result, scientists have to depend on atomic-level simulations that demonstrate how and where exactly this chemistry takes place.
The model demonstrates that the formation of nanodiamonds requires a delicate balance of pressure and temperature evolution.
If the initial pressure of detonation is very low, carbon solids - not diamonds - will form.
On the other hand, if the detonation pressure is too high, the carbon “seeds” of nanodiamonds will become contaminated by nitrogen, oxygen, or other elements, which inhibit the transition to diamond.
For more than five decades, researchers have known that detonations are responsible for the formation of nanodiamonds.
Depsite this, for the last the two decades, the atomic-level details of the formation of nanodiamonds have remained an open question.
The detonation of carbon-rich organic high explosives is the most common industrial method for the synthesis of nanodiamonds. Asteroid impacts on Earth or explosive volcanic eruptions can also result in the natural formation of nanodiamonds.
"Our work shows that the right path seems to be a high initial pressure followed by a sharp pressure decrease,” stated Bidault.
The research is the hypothetical part of a more global project supported by the French National Research Agency (ANR), involving the French-German Research Institute of Saint-Louis (ISL) and the French Alternative Energies and Atomic Energy Commission (CEA).