Jan 10 2019
Kanazawa University researchers have revealed that self-sorting is exhibited by certain hexagonal and pentagonal organic molecules. The study has been reported in Communications Chemistry.
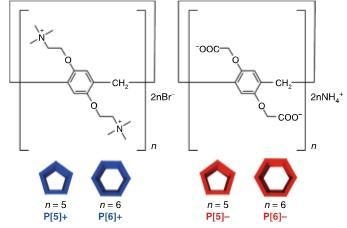 Structures of pillar[n]arenes, n = 5 and 6. Left, blue: cationic (positively charged) variants; right, red: anionic (negatively charged) variants. (Image credit: Kanazawa University)
Structures of pillar[n]arenes, n = 5 and 6. Left, blue: cationic (positively charged) variants; right, red: anionic (negatively charged) variants. (Image credit: Kanazawa University)
The effect can be used for growing multilayered tubular structures that maintain the initial cavities’ geometry.
Supramolecular assemblies are actually nanostructures ensuing from molecules that bind together into larger units through intermolecular interactions. Self-sorting, in which molecules detect copies of themselves and bind with them, is one method for controlling supramolecular assembly.
The findings of an interdisciplinary association between the Supramolecular group (Tomoki Ogoshi and coworkers) and the Atomic Force Microscopy (AFM) group (Hitoshi Asakawa, Takeshi Fukuma, and coworkers) of the Nano Life Science Institute (WPI-NanoLSI) Kanazawa University have now revealed that self-sorting behavior can emerge from the principle of geometrical complementarity by shape: in a combination of certain hexagonal and pentagonal molecular building blocks, hexagons bind to hexagons and pentagons bind to pentagons, and mixing no longer occurs.
The AFM group, which included Asakawa and members, performed experiments with molecules known as pillar[n]arenes, with n = 6 and n = 5, respectively corresponding to hexagonal and pentagonal shapes. Both of these molecules come in two “flavors”: negatively charged (anionic) or positively (cationic). The polygonal molecules are fundamentally rings of 5 or 6 matching organic units, each possessing a benzene ring; however, for the anionic and cationic variants, the composition of the units is different.
The Supramolecular group, which included Ogoshi and his colleagues, allowed cationic pillar[5]arenes (P[5]+ in shorthand notation) to adsorb on a quartz substrate. The team was able to grow P[5]+/P[5]–/P[5]+/… multilayers from this structure, by submerging it alternatingly in cationic and anionic pillar[5]arene solutions. Through ultraviolet-visible spectroscopy measurements, the addition of a layer was validated each time. The ensuing overall structure is a “nanomat” of tubular structures that have pentagonal pores. Analogous results were achieved for the pillar[6]arenes: it is possible to fabricate stacks of alternating anionic and cationic layers of the hexagonal molecules. Professors Tomofumi Tada and Takanori Fukushima and also co-workers from Tokyo Institute of Technology collaborated to investigate the arrangement of pillar[n]arenes on a surface.
To the researchers’ surprise, hexagonal and pentagonal building blocks could not be stacked when attempting to create a cationic layer on an anionic one, and vice versa. This is nothing but a manifestation of self-sorting: even if ionic interactions fuel the formation of cation-anion layered structures, only like polygons have the ability to self-assemble.
Furthermore, the team studied the structure of the first layer of P[6]+ or P[5]+ molecules on the quartz substrate. The two-dimensional (2D) packing structure did no display long-range structural order for the hexagonal molecules, while it did for the pentagonal molecules. This is partly due to the latter’s lower density. The same trend was noticed in the multilayer “nanomats”, that is, long-range order for the pentagonal stacks. A Monte Carlo simulation, through association with Professor Tomonori Dotera from Kindai University, simulated the ring shape-dependent packing structures.
The self-sorting effect detected by Ogoshi and coworkers holds immense potential applications.
According to the researchers, “The ultimate challenge will be to propagate cavity-shape information on the surface to provide shape-recognisable adsorption and adhesive materials.”