Jun 26 2019
Acute kidney injury (AKI) frequently complicates the treatment results of hospitalized patients, resulting in unsafe levels of hazardous chemicals collecting in the blood and causing many fatalities every year.
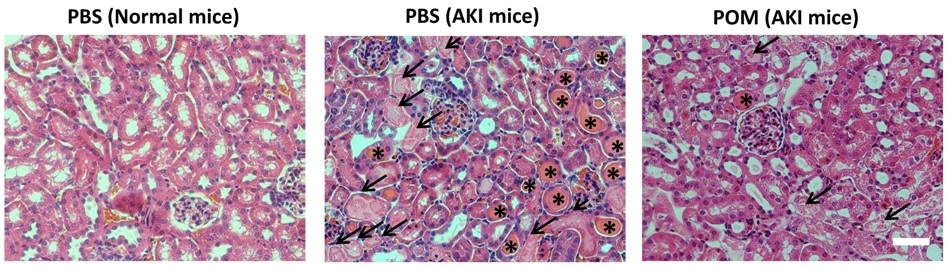 H&E staining of kidney tissues revealed that the ultra-small POM nanoclusters could recover renal function in the AKI mice. (Image Credit: D. Ni and W. Cai, et al., Department of Radiology, University of Wisconsin–Madison, Madison, WI.)
H&E staining of kidney tissues revealed that the ultra-small POM nanoclusters could recover renal function in the AKI mice. (Image Credit: D. Ni and W. Cai, et al., Department of Radiology, University of Wisconsin–Madison, Madison, WI.)
At present, only supportive treatment is available for AKI, but two associated research studies presented at the 2019 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging provide hope for treatment and prevention that is effective.
Reactive oxygen species (ROS), free radicals that activate oxidative stress and inflammation, are said to induce AKI. Mouse models were used in both studies to experiment with the new promising AKI therapies that target ROS.
The first research created minute nanoclusters with antioxidative properties that can pass via the kidneys’ glomerular filtration barrier to treat AKI.
“In this study, ultra-small sized molybdenum-based polyoxometalate (POM) nanoclusters with preferential renal uptake were found to act as a novel type of nano-antioxidant to efficiently alleviate clinical symptoms of AKI mice,” explains Dalong Ni, PhD, at the University of Wisconsin-Madison.
POM radiolabeled with zirconium-89 (89Zr) was intravenously injected into AKI mice. Longitudinal PET imaging was then carried out at different time points to track the renal accumulation in vivo. The therapeutic value was assessed by dynamic positron emission (PET) scans with gallium-68 (68Ga)-EDTA, H&E staining of kidney tissues, blood urea nitrogen and creatinine measurements, and biomarkers detection.
Ni reports, “The role of POM nanoclusters acting as antioxidants was confirmed both in vitro and in vivo, which was attributed to the readily variable valence state of molybdenum ions. Kidney transplantation and supportive therapies, such as rehydration and dialysis, are frequently required for patients with AKI. The protective effect of POM nanoclusters against AKI in living animals suggests exploring their use for the treatment of AKI patients.”
POM clusters could be synthesized in an easy, fast, and large-scale process, and they are mainly excreted through the kidneys (like most clinically-used imaging agents), making them highly biocompatible and reducing the potential toxic effects on patients.
Dalong Ni, PhD, University of Wisconsin-Madison
The second study was also carried out in the University of Wisconsin-Madison and uses very small nanomaterials as well. It shows that selenium-doped carbon quantum dots can both treat and prevent AKI by localizing in the kidneys and removing ROS.
For the research, carbon quantum dots with high antioxidant capacity were made by doping selenium (SeCQDs) through a hydrothermal treatment. Molecular imaging offers the most effective technique for examining the biodistribution of nanomaterials, so PET imaging of SeCQDs was preformed to assess biodistribution using 89Zr after functionalizing with deferoxamine.
The results of the SeCQDs treatment of the mice were compared with those from administration of an equal dose of amifostine, an FDA-permitted drug for AKI therapy.
The PET imaging results revealed that SeCQDs possess fascinating nano-bio interactions. Surprisingly, we found that the administered dose almost entirely accumulated in the kidneys or was rapidly excreted in the urine, with little present in the liver or other organs. Results similar to this have rarely been reported.
Zachary Rosenkrans, Pharmaceutical Sciences Department, University of Wisconsin-Madison
The high buildup of SeCQDs in the kidneys demonstrated a very effective therapy for the treatment of AKI that was induced using 50% glycerol, as well as prevention of AKI from cisplatin because of its antioxidant properties. In the same animal models, amifostine was ineffective in treating AKI.
Rosenkrans highlights, “Due to this, we were able to demonstrate the advantages of utilizing nanomaterials compared to small molecules for renal disease treatment—in this case, AKI. This could most directly benefit the one in five hospitalized patients that experience AKI.”
Enthusiastic about subsequent steps, he adds, “This work could lead to guidelines to tailor nanoparticles to target and be retained in the kidneys, which has transformative potential to treat renal diseases. This may provide new avenues for more effective delivery of drugs to kidney cancer (or other kidney diseases, such as glomerulonephritis) and thus more efficacious therapies.”