Jan 29 2020
Iron nanowires coated with drugs can offer a better option for cancer therapy as they can be steered to the tumor site by an external magnetic field, before starting a three-step cancer-killing process.
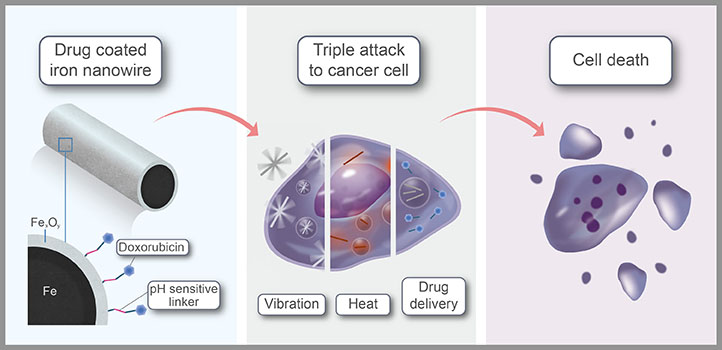 By combining low-power magnetic fields, which agitate nanowires, with laser heating and drug delivery, target cells can be killed efficiently. Image Credit: KAUST.
By combining low-power magnetic fields, which agitate nanowires, with laser heating and drug delivery, target cells can be killed efficiently. Image Credit: KAUST.
KAUST scientists jointly produced these nanowires that can release their drug cargo in cancer cells and can pierce holes in the cell membrane and supply a blast of heat, all at the same time. Although the combination therapy increases cancer cell death, due to its highly targeted nature, it should reduce side effects.
According to Jürgen Kosel, iron was evidently the material of choice to produce the nanowires. Kosel led the research team at KAUST, which includes Jasmeen Merzaban and Boon Ooi, and co-led the work with scientists from CIC biomaGUNE in San Sebastian, Spain.
The primary concern is safety. “Iron, in molecular form, is a native material in our bodies, essential for oxygen transport,” Kosel described. The nanowires include an iron core, coated with an iron oxide shell. He added, “Iron-oxide-based nanomaterials have been approved by regulatory bodies for use in magnetic resonance imaging and as a dietary supplement in cases of nutrition deficiency.”
Besides their biocompatibility, the magnetic properties of iron-based materials are very advantageous.
Using harmless magnetic fields, we can transport them; concentrate them in the desired area; rotate or make them vibrate, such as we did in this study; and even detect them through magnetic resonance imaging.
Aldo Martínez-Banderas, Research Team Member, KAUST
The researchers used low-power magnetic fields to agitate the nanowires in such a way that it opened the membrane of the target cells, causing cell death.
Another benefit is that core-shell nanowires get heated up as they strongly absorb near-infrared light. Light at this wavelength can enter far into the body; therefore, the nanowires could be heated with lasers oriented at the tumor site.
The core−shell nanowires showed an extremely high photothermal conversion efficiency of more than 80 percent, which translated into a large intracellular heat dose.
Aldo Martínez-Banderas, Research Team Member, KAUST
Lastly, the nanowires were coated with doxorubicin (an anticancer drug) through linkers that are sensitive to pH changes. Since the tumor environment is generally more acidic than healthy cells, the linker will selectively break down in or near cancer cells, releasing the drug where it is required.
“The combination of treatment resulted in nearly complete cancer cell ablation and was more effective than individual treatments or the anticancer drug alone,” stated Martínez-Banderas.
Taken together, the capabilities of iron-based nanomaterials make them very promising for the creation of biomedical nanorobots, which could revolutionize healthcare. While this might seem futuristic, the developments are well on their way.
Jürgen Kosel, Study Group Leader, KAUST