Jun 8 2020
At the Shinshu University, a research group under the guidance of Ryusuke Futamura analyzed the response of magnetic ionic liquids (MIL) to magnetic fields from the microscopic viewpoints.
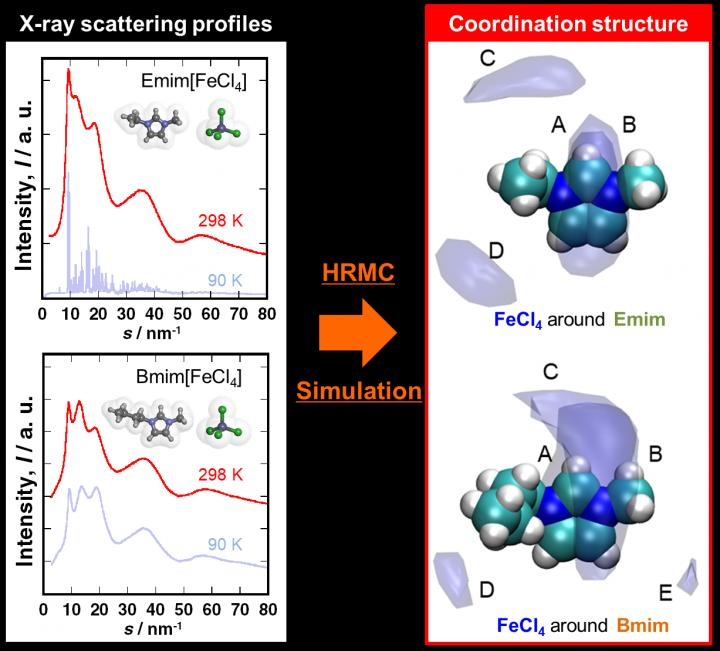 Magnetic ionic liquid structures were elucidated through hybrid reverse Monte Carlo simulation. The research results elucidated fundamental understanding of pure liquids with magnetic responses as well as lead to the development of MIL for a variety of practical applications. Image Credit: Ryusuke Futamura, Faculty of Science, Department of Chemistry, Shinshu University.
Magnetic ionic liquid structures were elucidated through hybrid reverse Monte Carlo simulation. The research results elucidated fundamental understanding of pure liquids with magnetic responses as well as lead to the development of MIL for a variety of practical applications. Image Credit: Ryusuke Futamura, Faculty of Science, Department of Chemistry, Shinshu University.
Magnetic fluids can be produced by dispersing ferromagnetic nanoparticles in a solvent and are sensitive to magnetic fields. Few pure liquids that are not mixtures also exhibit sensitivity to magnetic fields.
For instance, oxygen can exist in a liquid state at about −200 °C and is attracted to magnets. As part of their study, the researchers analyzed pure magnetic ionic liquids Bmim [FeCl4] and Emim [FeCl4] on the microscopic scale.
At room temperature, such liquids are attracted to magnets, but Emim also experiences a transition from paramagnetic to antiferromagnetic behavior at a temperature of 3.8 K.
Magnetic ions or atoms contain magnetic dipoles (north and south) in the molecular scale. These ions or atoms interact with each other and exhibit ferro- or antiferro-magnetism across a long distance in their crystal structures.
Bmim [FeCl4] is formless or amorphous and does not crystallize even at low temperatures. In this study, it was demonstrated that even in the amorphous state, an aligned association structure is formed by various magnetic ions and there is structurality in the short range.
This was the cause of the negative Curie-Weiss temperature, which can be viewed as a macroscopic physical property.
Analyzing and gaining insights into the formation of the liquid structure of Bmim[FeCl4] and Emim[FeCl4] was challenging. The amorphous objects and liquids do not exhibit a long-range ordered structure, denoting that analysis of the structure of these materials is carried out via X-ray scattering measurements and radial distribution analysis.
But MILs are binary systems that include anions and cations, due to which they are challenging to analyze by ordinary radial distribution analysis. To this end, the hybrid reverse Monte Carlo (HRMC) method proved helpful. By combining molecular simulation with X-ray scattering measurement, it evidently showed the accurate coordination structures of both the MILs.
Thus, it has been possible to discuss the cation-anion, anion-anion, and cation-cation of the liquid structure.
Visualization of the ion coordination structure has been feasible by using spatial distribution function analysis. The spatial distribution function’s temperature dependence illustrating the coordination structure of anions around the cations in the MIL demonstrates that when the temperature is lower, the coordination sphere is wider and the site is more blurred.
The scientists could clarify the characteristics of substances that occur in macroscopic physical properties from a microscopic viewpoint.
The study’s first author Futamura specializes in the nanospaces of porous materials. Futamura believes new composite materials can be produced by integrating ionic liquids and porous materials.
By limiting MIL in the nanospace of porous materials, Futamura believes new functional materials can be developed for several applications. Such MILs are regarded as organic-inorganic hybrid functional materials that could find excellent physical and chemical applications.
Journal Reference:
Futamura, R., et al. (2020) Configurational evidence for antiferromagnetic interaction in disordered magnetic ionic liquids by X-ray scattering-aided hybrid reverse Monte Carlo simulation. Journal of Molecular Liquids. doi.org/10.1016/j.molliq.2020.113321.