May 24 2021
A majority of commercially available chemicals are created with the help of catalysts. Generally, such catalysts contain very small metal nanoparticles that are positioned on an oxidic support.
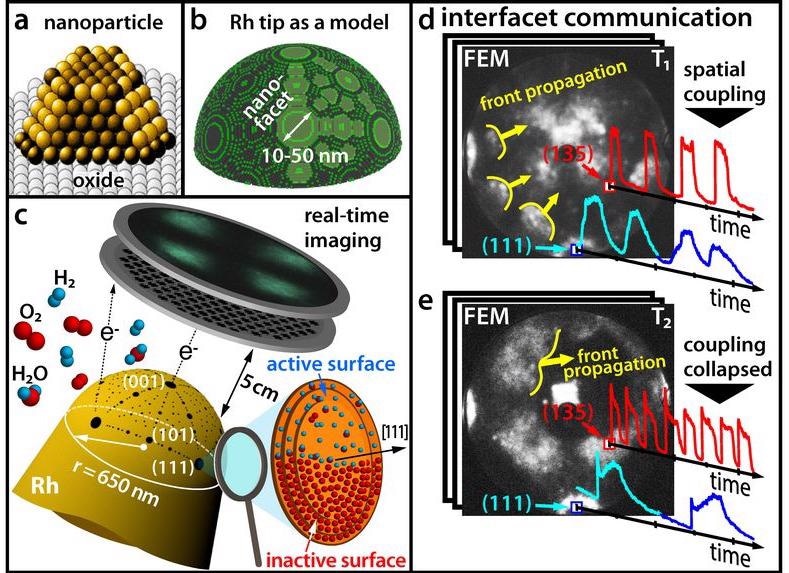 (a) Modern catalysts consist of nanoparticles. (b) A Rhodium tip as a model for a nanoparticle. (c) Tracing a chemical reaction in real time with a field emission microscope. (d) At low temperatures, different facets oscillate in sync. (e) At higher temperatures, synchronicity is broken. Image Credit: Vienna University of Technology.
(a) Modern catalysts consist of nanoparticles. (b) A Rhodium tip as a model for a nanoparticle. (c) Tracing a chemical reaction in real time with a field emission microscope. (d) At low temperatures, different facets oscillate in sync. (e) At higher temperatures, synchronicity is broken. Image Credit: Vienna University of Technology.
A catalytic nanoparticle is analogous to a cut diamond, the surface of which contains varied facets that are oriented in various directions. Besides this, the nanoparticle has crystallographically different facets—and such facets can have varied chemical characteristics.
To date, such variations have not been typically considered in catalytic studies because it is not easy to concurrently achieve data about the surface structure of the catalyst and about the chemical reaction itself.
At the Vienna University of Technology (TU Wien), scientists have now achieved this feat by integrating different microscopic techniques—using field ion microscopy and field electron microscopy, hydrogen oxidation on a solitary rhodium nanoparticle was effectively visualized at nanometer resolution and in real time.
This demonstrated unexpected effects that should be considered in the quest for more improved catalysts in the days to come. The results of the study have now been described in the scientific journal Science.
The Rhythm of Chemical Reactions
In certain chemical reactions, a catalyst can periodically switch back and forth between an active and an inactive state. Self-sustaining chemical oscillations can occur between the two states—the chemist Gerhard Ertl received the Nobel Prize in Chemistry for this discovery in 2007.
Günther Rupprechter, Professor, Institute of Materials Chemistry, Vienna University of Technology
This is also the case with respect to rhodium nanoparticles, which are utilized as a catalyst for the oxidation of hydrogen—the basis of each fuel cell. Under certain conditions, the nanoparticles can oscillate between a state where oxygen molecules dissociate on the particle surface and a state where hydrogen is attached.
Incorporated Oxygen Changes the Surface Behavior
When a rhodium particle is exposed to an atmosphere of oxygen and hydrogen, the oxygen molecules are split into individual atoms at the rhodium surface. These oxygen atoms can then migrate below the uppermost rhodium layer and accumulate as the subsurface oxygen there.
Yuri Suchorski, Study First Author and Professor, Institute of Materials Chemistry, Vienna University of Technology
Through hydrogen interaction, the preserved oxygen atoms can be subsequently brought out again and can be allowed to react with hydrogen atoms. Also, there is again space for more numbers of oxygen atoms within the rhodium particle and the cycle begins again.
“This feedback mechanism controls the frequency of the oscillations,” added Professor Suchorski.
To date, it was assumed that such chemical oscillations invariably occurred synchronously in the same rhythm across the whole nanoparticle.
In the end, the chemical processes that take place on the different facets of the surface of the nanoparticles are spatially paired, because the hydrogen atoms can easily transfer from one facet to the neighboring facets.
But the results reported by the research team of Professor Günther Rupprechter and Professor Yuri Suchorski revealed that things are, in fact, relatively more complicated: Under specific conditions, the spatial coupling is lifted and neighboring facets abruptly oscillate with considerably different frequencies—and in certain nanoparticle regions, such oscillating 'chemical waves' no longer propagate.
“This can be explained on an atomic scale. Under the influence of oxygen, protruding rows of rhodium atoms can emerge from a smooth surface,” added Professor Suchorski.
Such rows of atoms can subsequently serve as a form of “wave breaker” and block hydrogen atoms from migrating from one facet to another—causing the facets to become decoupled. If that is the case, the separate facets can create oscillations of varied frequencies.
On different facets, the rhodium atoms are arranged differently on the surface. That’s why the incorporation of oxygen under the differing facets of the rhodium particle also proceeds at different rates, and so oscillations with different frequencies result on crystallographically different facets.
Günther Rupprechter, Professor, Institute of Materials Chemistry, Vienna University of Technology
A Hemisphere Tip as a Nanoparticle Model
The key to decoding this complex chemical behavior lies in utilizing a fine rhodium tip as a prototype for a catalytic nanoparticle. Electrons can exit the tip upon applying an electric field and also because of the quantum mechanical tunneling effect.
Such electrons are expedited in the electric field and collide with a screen, in which a projection image of the tip is subsequently produced with about 2 nm resolution.
Unlike scanning microscopies, where the sites of the surface are scanned sequentially, this parallel imaging observes all surface atoms at the same time—otherwise, the desynchronization and synchronization of the oscillations will not be possible to track.
This latest understanding of the interaction of separate facets of a nanoparticle can presently result in more effective catalysts and offer better atomic insights into the mechanisms of spatial coupling, pattern formation, and non-linear reaction kinetics.
The study was financially supported by the Austrian Science Fund (FWF) as part of the Project “Spatial-temporal phenomena on surface structure libraries.”
Oszillierende chemische Reaktionen
Oscillating chemical reactions. Video Credit: Vienna University of Technology.
Journal Reference:
Suchorski, Y., et al. (2021) Resolving multifrequential oscillations and nanoscale interfacet communication in single-particle catalysis. Science. doi.org/10.1126/science.abf8107.