Reactive oxygen species (ROS) exhibit the potential to act as signal carriers during the development of malignant tumors. At a suitable concentration, ROS tends to mediate cell growth and signal transduction.
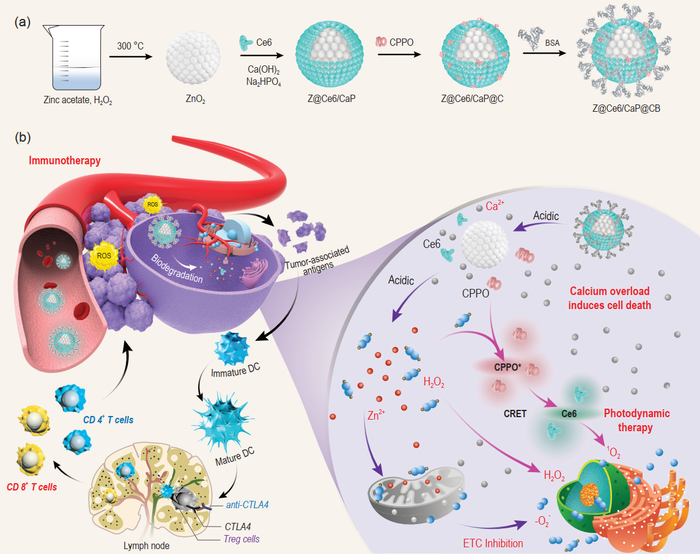 (a) Synthesis process of ZnO2@Ce6/CaP@CPPO/BSA (Z@Ce6/CaP@CB). (b) Schematic illustration of the mechanism of Z@Ce6/CaP@CB-based synergistic therapy. Z@Ce6/CaP@CB nanobombs can trigger multiple ROS storms (H2O2, 1O2, and O2-) and Ca2+ overload-induced cell death through a cascade reaction without external energy activation and effectively activate the systemic immune response while inhibiting the growth of primary tumors. Image Credit: ©Science China Press.
(a) Synthesis process of ZnO2@Ce6/CaP@CPPO/BSA (Z@Ce6/CaP@CB). (b) Schematic illustration of the mechanism of Z@Ce6/CaP@CB-based synergistic therapy. Z@Ce6/CaP@CB nanobombs can trigger multiple ROS storms (H2O2, 1O2, and O2-) and Ca2+ overload-induced cell death through a cascade reaction without external energy activation and effectively activate the systemic immune response while inhibiting the growth of primary tumors. Image Credit: ©Science China Press.
But ROS are known to be a double-edged sword. On becoming surplus, ROS could oxidize proteins, cause damage to the DNA structure and cause cell apoptosis. Furthermore, ROS can induce inflammation at the tumor site, which additionally enhances tumor immunogenicity.
Thus, raising the content of ROS in tumor sites has turned out to be an efficient technique for cancer therapy. Currently, the methods to produce ROS via external stimulations, like radiation sensitization, sonodynamic reaction and photodynamic reaction, are heavily restricted by the laser’s penetration depth, irradiation range of external excitation and safety-related issues of the radiation.
As a result of such issues, chemodynamic therapy has advanced, garnering extensive attention. Chemodynamic therapy makes use of surplus H2O2 in the tumor microenvironment without the need for external energy stimulation to produce ROS via the Fenton reaction.
But the existing therapeutic effect of chemodynamic therapy is considered to be unsatisfactory. This is because the initiation of an efficient Fenton reaction needs surplus H2O2 and rough acidic conditions. Besides exogenous ROS production approaches, increasing the generation of endogenous ROS to curb tumor growth is another potential technique.
Hindering the mitochondrial electron transport chain could improve the generation of ROS. But treating cancer only through increasing endogenous ROS is unsatisfactory, as it is hard to efficiently impede tumor growth with a limited amount of generated endogenous ROS.
Thus, in the field of cancer therapy, it is hard to create approaches for the selective generation of sufficient ROS in the absence of external energy stimulation under mild in vivo conditions.
In the latest research article reported in the Beijing-based National Science Review, researchers from the Changchun Institute of Applied Chemistry of the Chinese Academy of Sciences, China, designed a cascade-responsive ROS generation device with a domino effect and in the absence of external stimulation for the particular generation of numerous severe ROS storms at the tumor site.
The co-authors of the study Yang Liu, Yinghui Wang, Shuyan Song, and Hongjie Zhang have discovered that the simple introduction of the synthesized ZnO2@Ce6/CaP@CPPO/BSA nanobomb into the tumor would induce a “domino effect.”
This in turn could activate the production of several ROS storms and Ca2+ overload, as well as efficiently activate the systemic immune response while hindering the growth of primary tumors. Furthermore, tumor metastasis can be efficiently avoided by adjuvant treatment with anti-CTLA4 checkpoint blockers.
This study received financial support from the National Natural Science Foundation of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, and the Youth Innovation Promotion Association of the Chinese Academy of Sciences.
Journal Reference:
Liu, Y., et al. (2021) Cascade-responsive Nanobomb with domino effect for anti-tumor synergistic therapies. National Science Review. doi.org/10.1093/nsr/nwab139.