Novel research has been published in the journal Nano Convergence on the regeneration of skeletal muscle tissue with the aim of using nanotechnology to guide muscle differentiation.

Study: Nano-sized graphene oxide coated nanopillars on microgroove polymer arrays that enhance skeletal muscle cell differentiation. Image Credit: Rattiya Thongdumhyu/Shutterstock.com
Here, researchers used nanotechnology to guide muscle differentiation with nano-sized graphene oxide (sGO)-modified nanopillars on microgroove hybrid polymer array (NMPA).
Solving Current Skeletal Degeneration Issues
Skeletal muscle has a critical function in body movements and breathing; muscle cells are contracted and released due to complex biochemical signals generated and transferred from the peripheral and nervous systems.
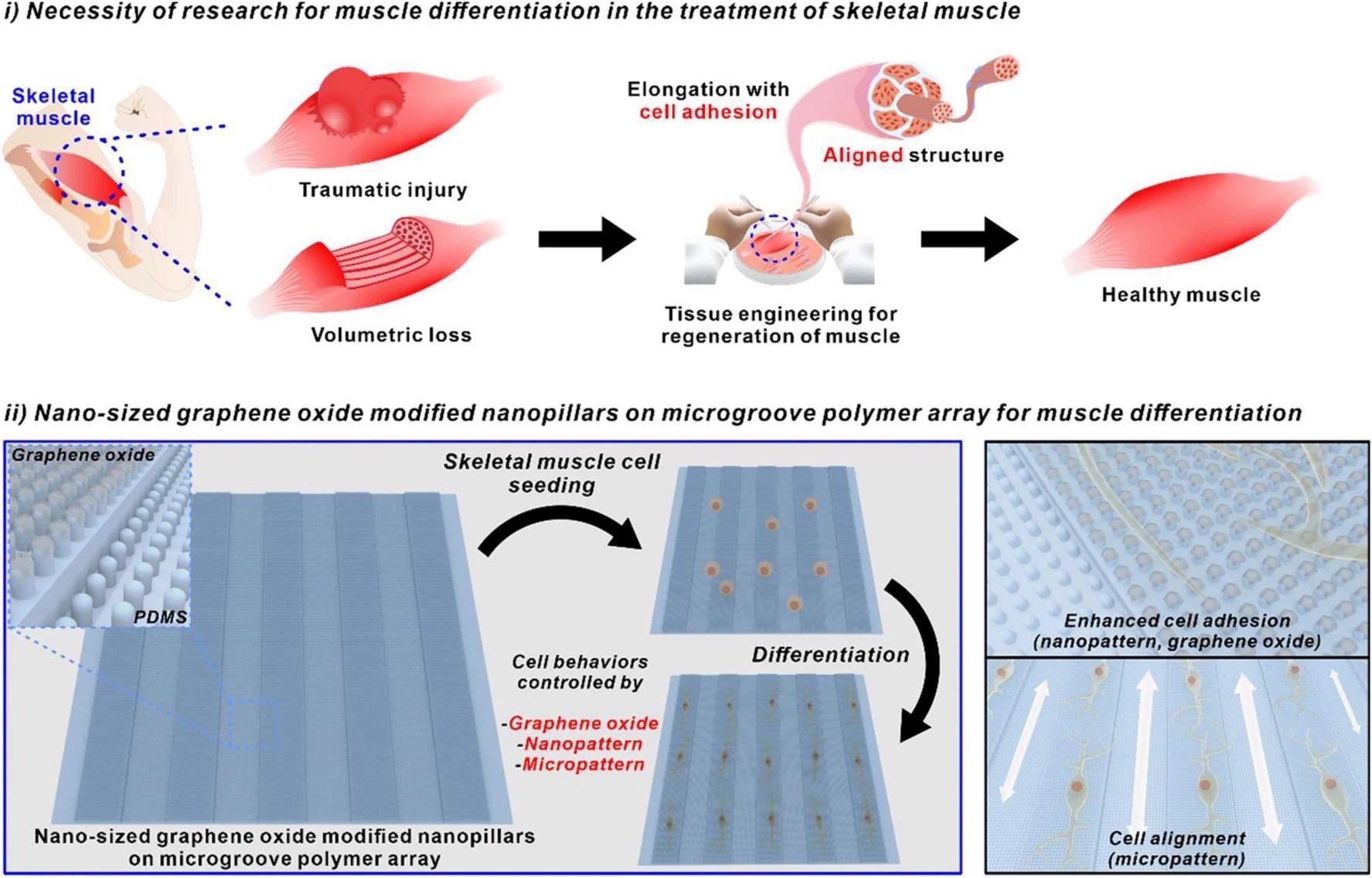
Utilizing nano-sized graphene oxide-modified nanopillars on microgroove polymer array for muscle differentiation. Image Credit: Choi, H., et al
Muscle tissue is predominantly composed of long, postmitotic multinucleated fibers, and maintenance of this tissue is mediated by satellite cells located closely.
Muscle satellite cells or satellite stem cells can be activated by stimuli such as physical trauma or growth signals resulting in their cell divisions, either increasing their number or leading to progenitors. The proliferation and differentiation of myogenic progenitors through fusion with each other or to damaged fibers enable the restoration of damaged fibers as well as their associated function.
This is significant for maintaining muscle health, which if lost can lead to numerous health problems, including chronic inflammatory diseases, cancer, and neurological disorders. Disease-related muscle atrophy and fatigue are significant as skeletal muscle weakness can result in increased hospitalization time, limitations in exercise, and overall poor quality of life.
While natural repair mechanisms may aid with small infractions in skeletal muscle health, with the loss of mass muscle bundles or damage from genetic abnormalities and mutations, the natural repair mechanism is ineffective.
Excitingly, skeletal muscle regeneration factors can be promising for solving the degeneration of muscle tissue.
Novel research has focused on in vitro muscle cell generation through controlling the differentiation of multipotent stem cells or unipotent pre-myogenic cells such as myoblasts.
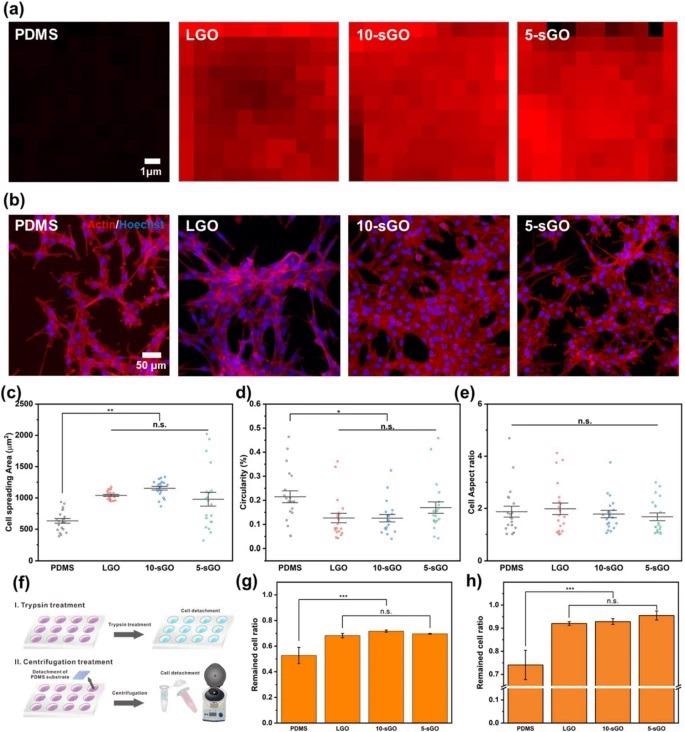
Confirmation of the effect of graphene oxide on cell behaviors. a FE-SEM images of PR nanohole patterns and PDMS nanopillar patterns, and b size distribution of PDMS patterns. Raman intensity map of the bare PDMS, LGO-, 10-sGO-, and 5-sGO-modified PDMS. b Confocal microscope images of the cells on bare PDMS, LGO-, 10-sGO-, and 5-sGO-modified PDMS immunostained with actin (Red) and Hoechst (Blue). c−e Cell spreading area (c), circularity (d), and cell aspect ratio (e) of the cells on bare PDMS, LGO-, 10-sGO-, and 5-sGO-modified PDMS. f Schematic diagram of trypsin and centrifugation treatment process. g, h Cell ratio remaining on the PDMS, LGO-, 10-sGO-, and 5-sGO-modified PDMS after trypsin (g) and centrifugation (h) treatment. (* p ≤ 0.5, ** p ≤ 0.01, *** p ≤ 0.001). Image Credit: Choi, H., et al
Inducing Skeletal Muscle Cell Differentiation
The most common method of inducing skeletal muscle cell differentiation involves using a cultivation medium containing various myogenic differentiation factors, including FGF, TGF-β, and IGF. This can potentially modulate the cellular microenvironment, including the extracellular matrix (ECM), and affect the state of cells.
An innovative research team has utilized nano-sized graphene oxide (sGO) of various sizes on a microgroove hybrid polymer array (NMPA) to investigate the most effective method of controlling skeletal muscle cell differentiation.
The incorporation of graphene oxide (GO) can increase cell adhesion levels on micropatterned polymeric arrays and nanopatterns through techniques such as scanning electron microscopy and Raman spectroscopy.
The above schematic diagram can illustrate how nano-sized graphene oxide-modified nanopillars on a microgroove polymer array can be used to seed skeletal muscle cells and allow for enhanced cell adhesion along with hybrid pattern arrays such as nanopatterns and micropatterns.
Three different parameters were investigated in this study: cell spreading, circularity, and aspect ratio of the myoblast cell line, C2C12. The efficiency of skeletal muscle cell differentiation of this cell line was evaluated using immunofluorescence.
This novel research has resulted in excitingly positive results, which enabled the enhancement of skeletal muscle cell fabrication. The hybrid pattern arrays utilized by the researchers were found to enhance cell spreading and alignment. Additionally, with the incorporation of graphene oxide on the hybrid pattern array, the differentiation of cells and stable cell culture on the polymer substrate was increased simultaneously.
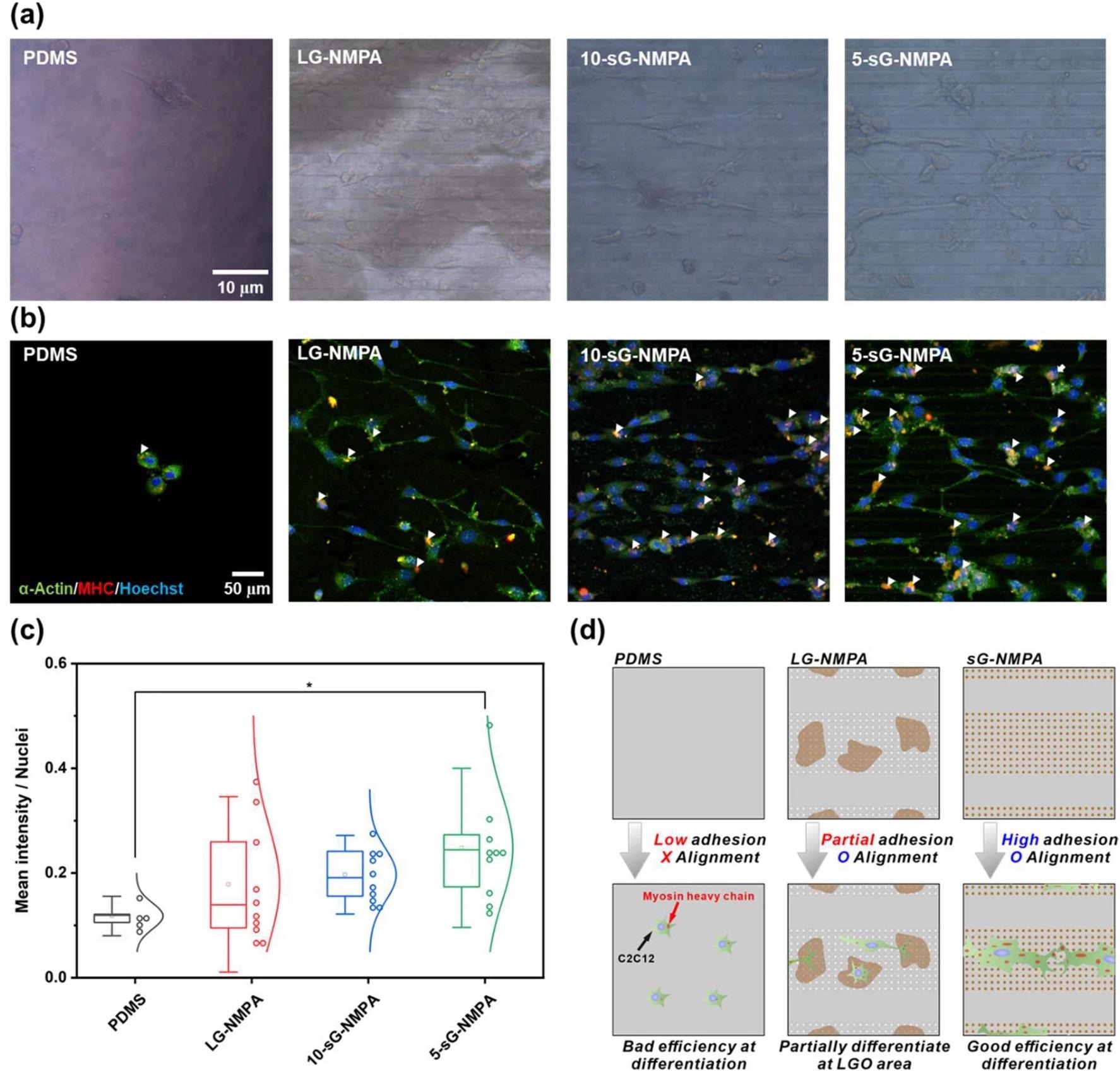
Confirmation of myogenic differentiation of the cells. a Brightfield images of the cells on the bare PDMS, LG-NMPA, 10-sG-NMPA, and 5-sGO-NMPA. b Confocal microscope images of the cells on bare PDMS, LG-NMPA, 10-sG-NMPA, and 5-sGO-NMPA immunostained with α-actin (Green), MHC (Red), and Hoechst (Blue). c The graph for mean intensity divided by number of nuclei. d Schematic diagram of the correlation between GO size and cell differentiation based on the mean intensity data. (* p ≤ 0.5). Image Credit: Choi, H., et al
The Future of Regeneration
This novel regenerative strategy utilizing GO-coated NMPA can be seen as an effective platform for skeletal muscle cell differentiation. This research is a critical step for furthering the field of regenerative medicine as well as biorobot technology that comprises muscle cells and polymer substrates.
Utilizing this research for tissue engineering provides a basis for further research into potentially novel therapies that can target skeletal muscle cell damage after injuries, as well as genetic mutations and disorders.
Enhancing the field of medicine in this manner can be revolutionary for tissue engineering and regeneration, and allows for patient care to be a priority, ensuring good quality of life for an aging population.
Continue reading: Oxidation-Reduction Processes for Graphene and Graphene Oxide Production.
Reference:
Choi, H., Kim, C., Lee, S., Kim, T. and Oh, B., (2021) Nano-sized graphene oxide coated nanopillars on microgroove polymer arrays that enhance skeletal muscle cell differentiation. Nano Convergence, 8(1). Available at: https://nanoconvergencejournal.springeropen.com/articles/10.1186/s40580-021-00291-6
Further Reading:
Dumont, N., Bentzinger, C., Sincennes, M. and Rudnicki, M., (2015) Satellite Cells and Skeletal Muscle Regeneration. Comprehensive Physiology, pp.1027-1059. Available at: https://onlinelibrary.wiley.com/doi/10.1002/cphy.c140068
Powers, S., Lynch, G., Murphy, K., Reid, M. and Zijdewind, I., (2016) Disease-Induced Skeletal Muscle Atrophy and Fatigue. Medicine & Science in Sports & Exercise, 48(11), pp.2307-2319.Available at: https://journals.lww.com/acsm-msse/Fulltext/2016/11000/Disease_Induced_Skeletal_Muscle_Atrophy_and.28.aspx
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.