Researchers at the University of Houston have demonstrated droplet size dependence in water to ice phase transitions on the surfaces of anodized aluminum oxide (AAO) nanomembranes. This research was published in the journal Nature Communications.

Study: Freezing of Few Nanometers Water Droplets. Image Credit: Mario7/Shutterstsock.com
At sub 10 nm scales, transformation temperature shows a strong dependence on water droplet size. At 2 nanometers, this temperature drops below the homogenous bulk nucleation limit.
In most natural and industrial environments, water to ice phase transitions occur on heterogeneous surfaces, that is, when water droplets are in contact with another medium.
Understanding this phenomenon will lead to exciting advances in biomimicking technology with critical applications in anti-icing systems for aviation, infrastructures and even cryopreservation systems.
A Quick Primer on Nucleation
Nucleation is the first step in the process of forming a new thermodynamic phase or a new structure via self-organization. It is typically characterized by the length of time needed for the new phase or structure to appear.
The primary nucleation time defines the length of time it takes for the first crystal nuclei to form. While secondary nucleation depends on the pre-existence of crystals to form, primary nucleation does not. Both primary and secondary nucleation are needed in crystal formation, but their mechanisms are different.
The process of nucleation has been found to be dependent on impurities within the system. Thus, we can distinguish between two types of nucleation: Homogeneous nucleation occurs away from a surface, whereas heterogeneous nucleus occurs on a surface.
In thermodynamically closed systems, the Gibbs free energy describes the maximum amount of non-expansion work that can be extracted from the system. Away from a surface, there is less free energy to form crystals, and therefore homogeneous nucleation is slower (and less common) than heterogeneous nucleation.
Lowering the Freezing Limit on Heterogeneous Nanosurfaces
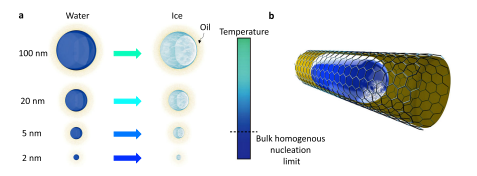
The length dependence of water–ice transformation in a heterogeneous mode. For 2 nm water droplets, heterogeneous nucleation could break the limit of bulk homogeneous nucleation. b Schematic of a nanodroplet formed in confined geometry and surrounded by an oil environment where ice nucleation occurs at the soft oil-water interface. The ice nucleation changes the local curvature of the oil-water interface. Image Credit: Hakimian, A., et al.
The researchers at the University of Houston have studied the formation of ice crystals in confined geometries down to 2 nm.
Water nanodroplets were formed inside pores of anodized aluminum oxide (AAO) membranes. The membranes had a diameter of 1 cm and a thicknesses ranging between 50 and 60 μm. The membrane thicknesses determined the length (size) of the pores while pore diameters ranged from 2 to 150 nm.
Except for 2 and 5 nm thick membranes, the pores were uniformly (isotropically) distributed along the thickness of the membrane: the 2 and 5 nm thick membranes comprised two layers with different pore dimensions.
Water nanodroplets were immersed in an octane oil environment so they could form a water-oil interface. Thus, ice nucleation could be initiated at this interface. Since the solid-liquid phase-change temperature of octane (at −57 °C) is well below the temperatures considered in the study, it would not influence the results observed.
As the electrical conductance of water and ice differ by three orders of magnitude, the team could detect water-ice phase transitions using a four-probe method. They also used Fourier-transform infrared spectroscopy (FTIR) spectroscopy to independently verify their results.
Using representative membranes with 150 nm pores, the team gradually decreased the temperature at a rate of 0.3 °C per minute and measured the current-voltage curves at each temperature.
As the temperature decreased, resistances across the pores increased linearly, showing the effect of temperature changes on the electrical resistivity. However, between −9 °C and −11 °C, a high, nonlinear shift in electrical resistance occurred. This jump indicated the water-ice phase transformation inside the pores.
The electrical conductance method was also used on membranes with pore sizes of 10, 20, 40 and 80 nm. Nonlinear shifts in electrical resistance were also detected but at different temperatures, showing the dependence of transformation temperatures on droplet size.
The team also observed that water-ice phase transformation at pore dimensions of 2 nm occurs at temperatures lower (−41 °C) than for homogeneous bulk nucleation (about −38 °C). Critically, they have shown that, at a few nanometer scales, the softly curved interface of oil-water plays a critical role in suppressing ice nucleation.
This opens up new opportunities for the study of ice formation on heterogeneous surfaces in industrial, medical and other environments.
Reference
Hakimian, A., et al., (2021) Freezing of few nanometers water droplets. Nature, [online] 12, 6973. Available at: https://www.nature.com/articles/s41467-021-27346-w
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.