Bioinspired by tunicates and mussels, a Korean research team has created a “bomb-like” anticancer therapeutic agent that only destroys cancer cells.
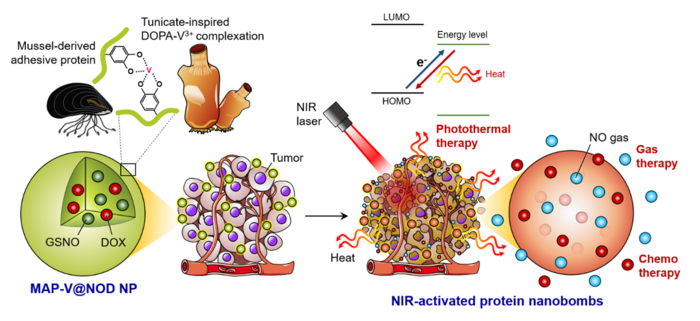 Schematic diagram of the mechanism of action of photoactivatable adhesive nanobombs made with bioinspired strategies from marine tunicates and mussels. (Image Credit: POSTECH).
Schematic diagram of the mechanism of action of photoactivatable adhesive nanobombs made with bioinspired strategies from marine tunicates and mussels. (Image Credit: POSTECH).
The nanobomb produces heat in precise sites where near-IR (NIR) laser is irradiated to create nitric oxide gas, which has an anticancer effect and concurrently discharges anticancer drugs to accomplish the trimodal treatment of photothermal-gas-chemo-therapy.
The POSTECH research team was headed by Professor Hyung Joon Cha and Dr. Yeonsu Jeong (Department of Chemical Engineering) in partnership with Professor Yun Kee Jo’s team at Kyungpook National University (Department of Biomedical Convergence Science and Technology, School of Convergence).
The researchers engineered a photoactivatable adhesive nanobomb with mussel adhesive proteins (MAPs) and the catechol-vanadium complex of tunicates that involves light-associated energy conversion through electron transfer. The term ‘photoactivatable’ denotes the characteristic where properties vary based on the external light.
Since cancer manifests via multiple biological pathways, a process that integrates several treatments rather than one single treatment is generally employed. But it is challenging to deliver several therapeutic agents at once to a particular cancer site because of the large amount of fluid found in the human body.
Among such approaches, light-triggered photothermal therapy (PTT) employs nanoparticles of metals, synthetic polymers or carbon. However, their weak biodegradability, which can cause a hazard of systemic toxicity, and low photothermal conversion efficiency — the efficiency at which the captured light is turned to heat — render effective cancer treatment tough.
To this, the researchers developed nanoparticles by incorporating the catechol-vanadium complexes — which help electrons and light travel in tunicates — to the MAP. When these nanoparticles are exposed to the light from NIR lasers, the temperature can be increased to 50 degrees within 5 minutes, displaying exceptional photothermal conversion efficiency of around 50%.
These nanoparticles can be retained on cancer cells for an extended period owing to the robust adhesiveness of MAP. Furthermore, the excellent biocompatibility and biodegradability of proteinic nanoparticles resolve the safety matter of traditional light-responsive nanotherapeutics.
When the nanoparticles comprising a thermo-sensitive nitric oxide (NO) donor and an anticancer drug were irradiated using the near-IR (NIR) laser, NO gas and drugs were effectively discharged due to the photothermal effect, verifying their likelihood for use in a nanobomb.
It is generally tough to retain the anticancer effects of NO gas as it rapidly biometabolizes. However, the nanobomb can regulate the release of gas with light, which significantly raises the delivery efficiency of NO.
In preclinical testing in animal models, the tumor grew back 15 days after being treated with only a single photothemal treatment, whereas the therapeutic effectiveness was extended with trimodal treatments using nanobombs as no tumors were seen for around 1 month.
With a single nanoparticle, various site-specific therapeutic agents can be locally administered and multimodal treatments can be easily controlled with a single stimulus, which will be effective in treating cancer patients in the future.
Professor Hyung Joon Cha, Department of Chemical Engineering, Pohang University of Science and Technology
“This nanobomb can be applied not only to photothermal-gas-chemo-therapy, but also to the delivery of therapeutics including genes and antibodies or contrast agents, and therefore can be widely used according to the specific needs of the patient or the condition,” Professor Hyung Joon Cha added.
This research was chosen as the front cover paper of the December issue of Advanced Healthcare Materials, a well-known journal in the domain of materials science.
The research was performed with the support from the National Research Foundation grant funded by the Ministry of Science and ICT of Korea, the National Research Foundation grant funded by the Ministry of Education of Korea, and the Marine BioMaterials Research Center grant funded by the Ministry of Oceans and Fisheries of Korea.
The mussel adhesive protein technology has gone through a technology transfer to Nature Glutech Co., Ltd., and clinical trials will take place after procuring investigational new drug (IND) approvals.
Journal Reference:
Jeong, Y., et al. (2022) Tunicate-Inspired Photoactivatable Proteinic Nanobombs for Tumor-Adhesive Multimodal Therapy. Advanced Healthcare Materials. doi.org/10.1002/adhm.202101212.