In a research study published recently in the journal Agriculture, an electrolytic sensor based on the concentration of floral nano-ZnO and the identification of immune reaction was created for the high-sensitivity monitoring of Tenuazonic acid (TeA) in fruit and vegetables.

Study: A High Sensitivity Electrochemical Immunosensor Based on Monoclonal Antibody Coupled Flower-Shaped Nano-ZnO for Detection of Tenuazonic Acid. Image Credit: Kateryna Kon/Shutterstock.com
When comparing various morphologies, dimensions, and crystalline structures of nano-ZnO, the researchers discovered that floral nano-ZnO (ZnO NFs) with a hexagonal phase had the best surface area and conductance.
What is Tenuazonic Acid (TeA)?
When compared to other Alternaria chemicals, tenuazonic acid (TeA) exhibits a high level of toxic effects, including neurotoxicity and probable carcinogenic effects.
It may also cause cumulative harm when combined with other toxins, leading to acute poisoning.
TeA can be found in a variety of foods, including grains, peppercorns, vegetables, fruits, and even animal proteins, with levels of contamination varying from a few milligrams to thousands of milligrams.
TeA was included by the United States National Council for Occupational Health & Safety in 1979 due to its high contamination of farm products.
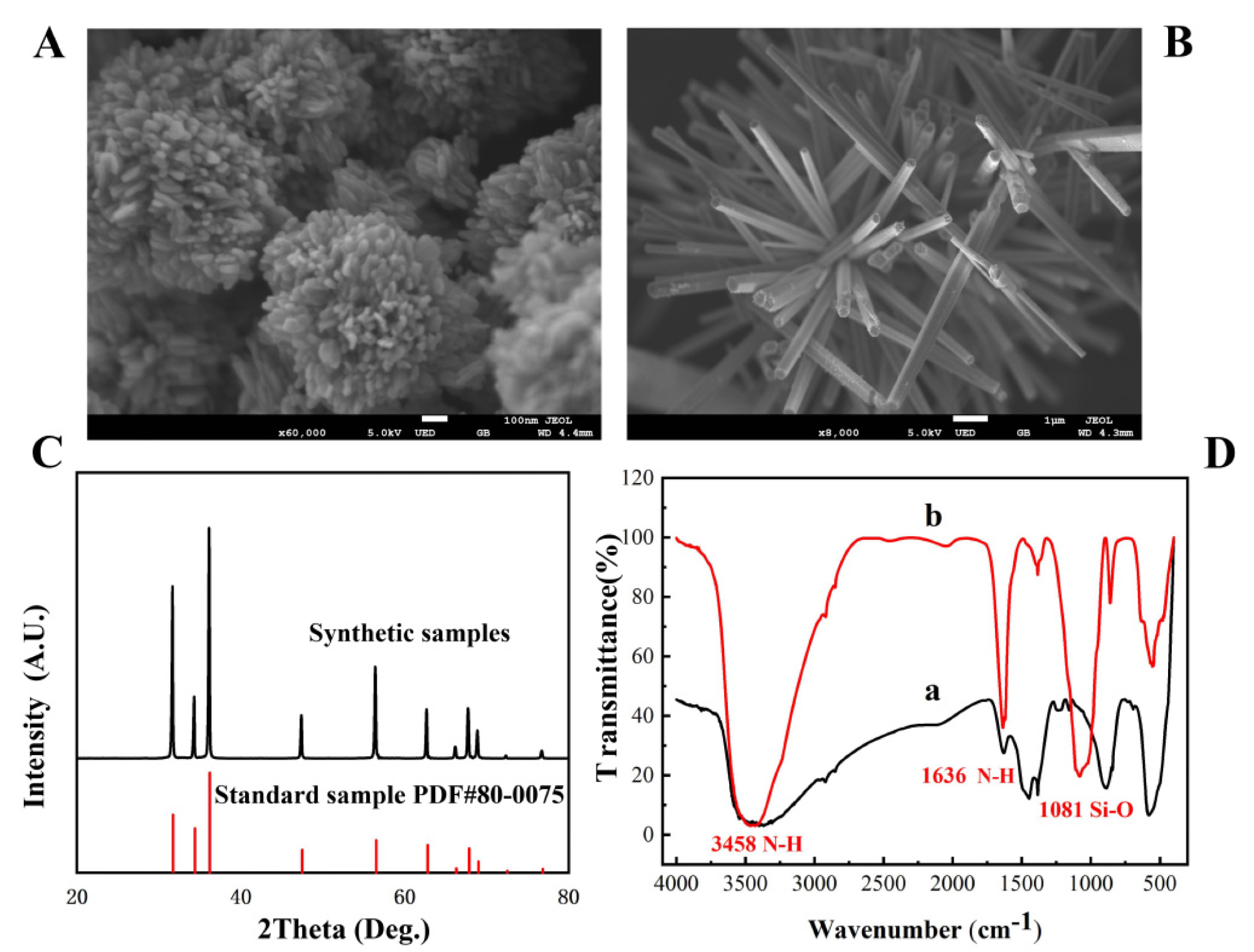
Figure 1. The SEM image of flower-shaped nano-ZnO (A) and brush-shaped nano-ZnO (B); XRD patterns of ZnO (C); FT-IR spectrum of amino-modified nano-ZnO (D). © Zhang, C. et al. (2022)
To date, enzyme-linked immunoassay (ELISA) and column chromatography spectroscopy have been the primary methods for detecting the carcinogen TeA.
However, since these traditional detection systems have significant drawbacks, such as being inconvenient, time-consuming, and having low susceptibility, enhancements in sensing methods of TeA are still needed.
Importance of ZnO Based Electrochemical Biosensors
In comparison to the approaches mentioned above, the electrochemical biosensor has been commonly regarded as a potent assessment technique with several advantages, including quick detection rate and mobile devices.
Recent research has focused on increasing its high specificity using a nanocomposite with a large surface area, great conductance, and superior photocatalytic characteristics.
Zinc oxide (ZnO) is a global semiconductor that may function as both a nanocomposite and a transistor. Because of its huge surface area, excellent durability, and superior cytocompatibility, it can offer much more redox potential for biorecognition components such as nucleic acid aptamers and monoclonal antibodies.
Since numerous microstructures of ZnO have been effectively implemented to biosensors to identify different bioactive molecules, it is essential to measure the impact of varying morphological features of ZnO on surface area and permeability to widen its implementation in delicate biosensors.
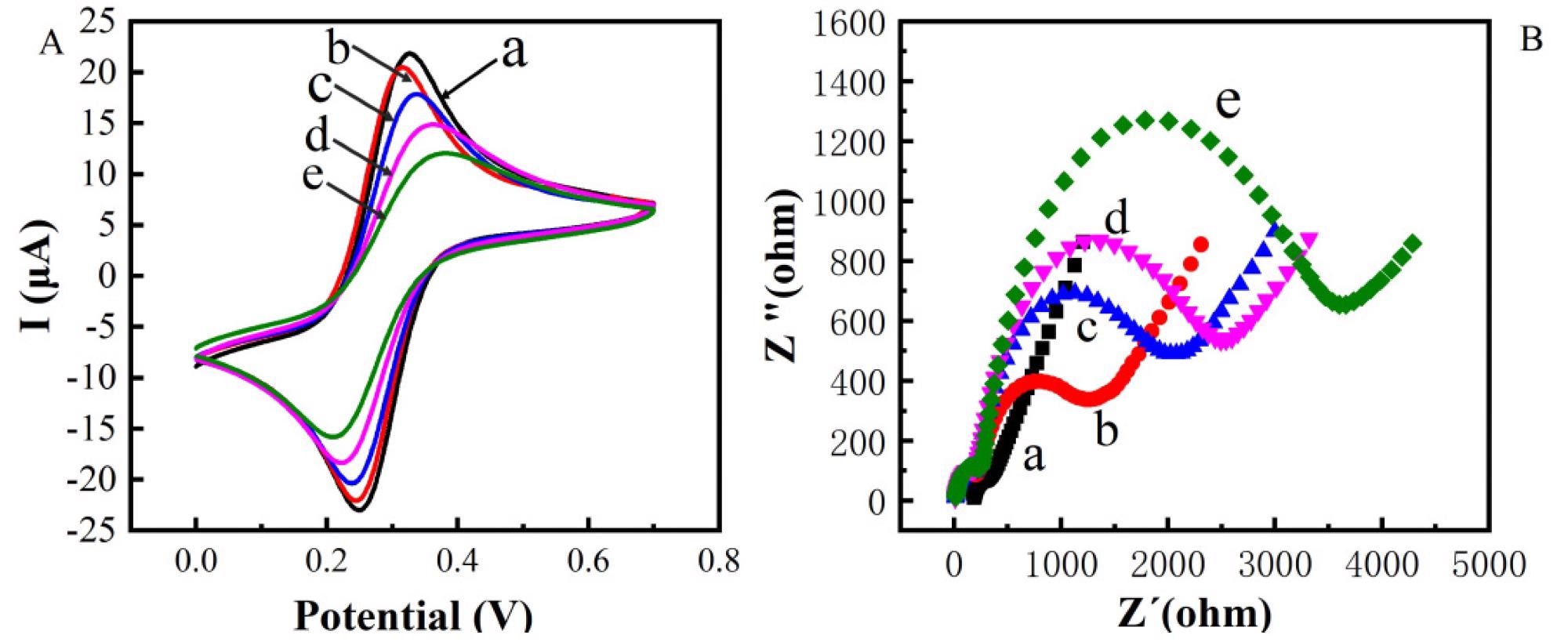
Figure 2. Cyclic voltametric characterization of constructing electrochemical immunosensor (A) Electrochemical impedance spectroscopy characterization of constructing electrochemical immunosensor (B) (a: bare Au; b: bare Au/ZnO; c: bare Au/ZnO/antibody; d: bare Au/ZnO/antibody/BSA; e: bare Au/ZnO/antibody/BSA/TeA). © Zhang, C. et al. (2022)
ZnO NFs as Biosensors for Detection of TeA
In this study, by carefully adjusting pH, response duration, activation time, and precipitator, ZnO with various morphologies, dimensions, and crystallinity was manufactured.
Because of the hard surface, three-dimensional structure, and numerous gaps on each nucleotide surface, the floral nanoscale ZnO has the highest load-bearing capacity.
Through an amidation process between the amino group of antigens and the carboxylate, the ZnO NFs were covalently bonded with TeA specific antibodies and subsequently anchored on a gold working electrode altered with 2-mercaptobenzoic acid (MBA).
Construction of Electrochemical TeA Sensor
A traditional three-electrode cell arrangement was used in which a gold wire acted as the electrode material, an Ag/AgCl electrode functioned as the reference electrode, and a Pt cable worked as the counter electrode.
The gold electrode was first soaked in a 1 percent MBA absolute ethanol, then maintained at 37 °C for 2 hours before being washed with distilled water and air-dried.
CV imaging and electrical resistance spectrometry were performed after each of the layers above had been modified.
The electrochemical behavior was investigated using a differentiated pulse voltammetry (DPV) test with a phase voltage of 4 mV, a frequency of 25 Hz, and an intensity of 25 mV.
Research Conclusion and Prospects
In conclusion, to overcome the high-loading issue of specific antibodies, the researchers created altered ZnO and floral nano-ZnO.
The effective production of Au/ZnO/antibody/BSA/TeA was validated by CV and EIS studies. Moreover, the electrolytic biosensor has good specificity and low resistance.
Sensitivity experiments revealed that it has a unique anti-interference capability against pollutants with a composition comparable to TeA.
TeA was effectively determined with a low detection limit using a straightforward, cost-effective, and pollution-free nano-ZnO, paving the way for the development of more economical and accurate biosensors for the identification of additional substances.
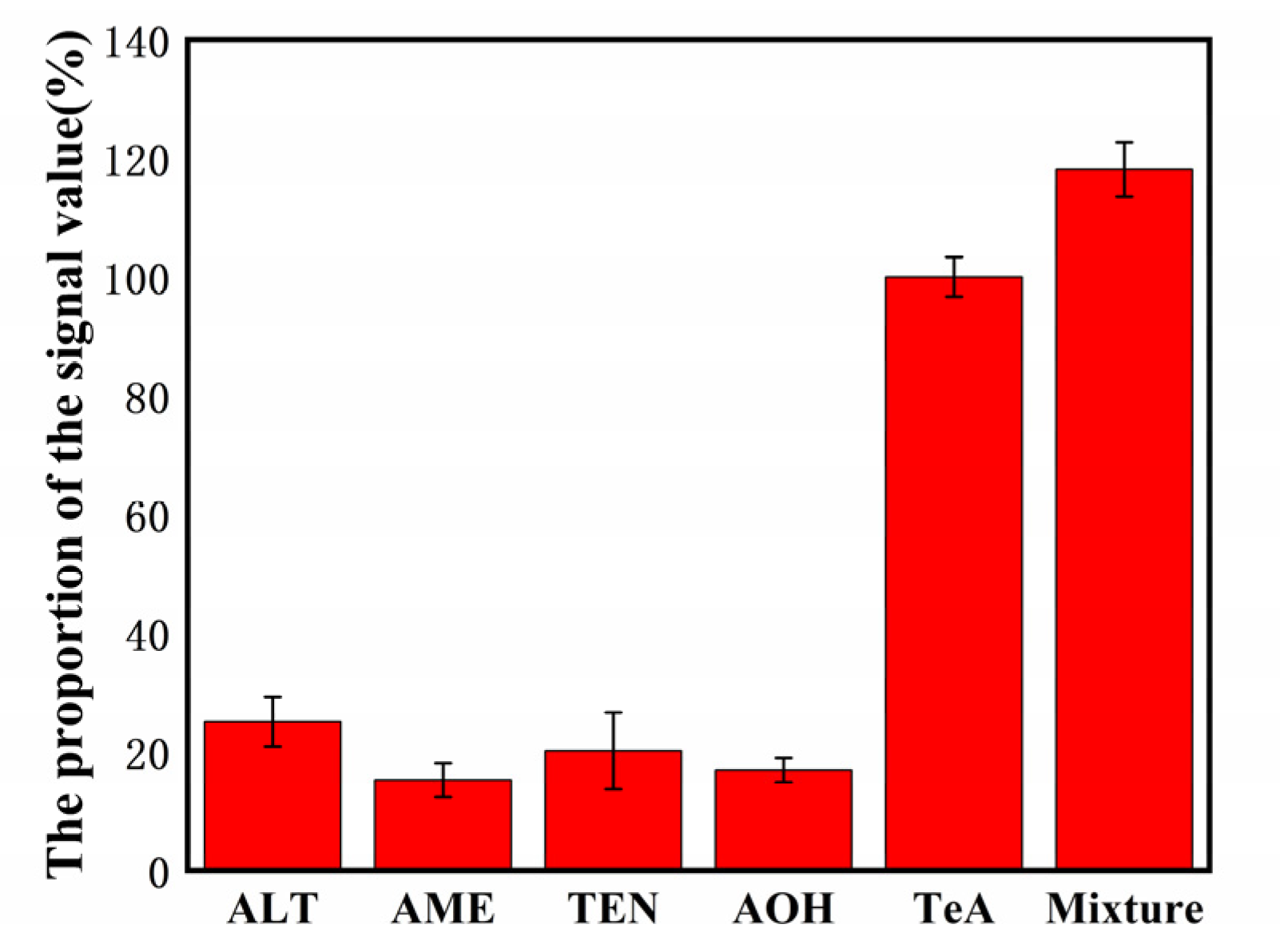
Figure 3. Selectivity of the biosensor detection of TeA (500 pg/mL) against the interference proteins: 50 ng/mL ALT, 50 ng/mL AME, 50 ng/mL TEN, and 50 ng/mL AOH. © Zhang, C. et al. (2022)
Reference
Zhang, C. et al. (2022). A High Sensitivity Electrochemical Immunosensor Based on Monoclonal Antibody Coupled Flower-Shaped Nano-ZnO for Detection of Tenuazonic Acid. Agriculture, 12(2), 204. Available at: https://www.mdpi.com/2077-0472/12/2/204.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.