Coordination nanosheets are newly evolving 2D materials with a wide variety of applications. However, extremely crystalline nanosheets are hard to synthesize through solution-based methods.
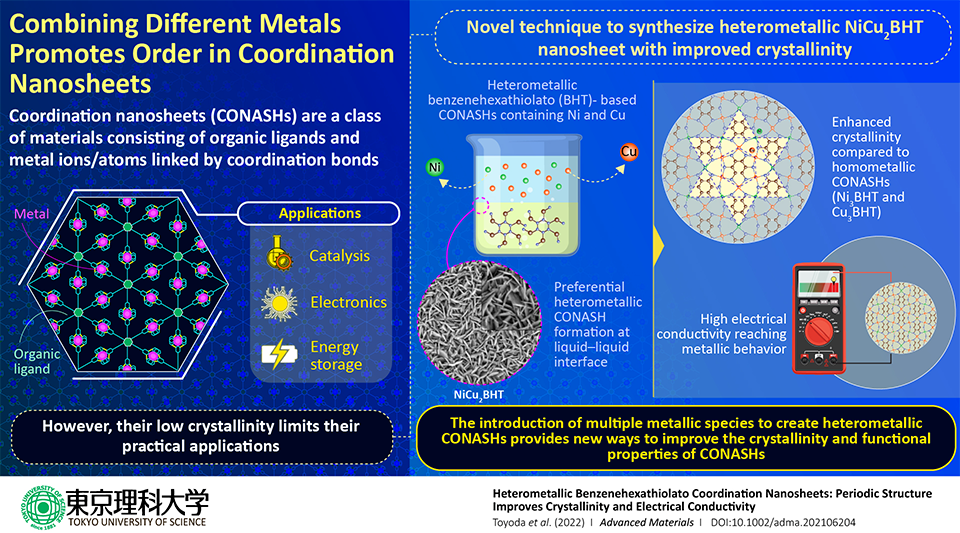
Image Credit: Tokyo University of Science.
In a new study, researchers discovered an uncomplicated strategy to enhance the structural order and performance of nanosheet films by using two diverse metal ions rather than one. Their findings focus on a solution for the progress of high-quality coordination nanosheets with excellent crystallinity and conductivity.
Coordination nanosheets are a new and upcoming family of two-dimensional (2D) materials, quickly gaining prominence in the domain of nanomaterials. They include metal ions and organic ligand molecules, connected to each other to produce one framework, via coordination bonds.
These nanosheets serve as building blocks, which can be mixed and matched to create a wide range of planar structures, with probable applications in batteries, electronic devices and catalytic systems.
In 2013, benzenehexathiolato (BHT) was revealed as a strong organic ligand in coordination nanosheets. It was noted that upon altering the element used in the metal centers, it is possible to form BHT-based nanosheets with enormously different structural features.
However, the production of BHT-based coordination nanosheets via solution-based processes has proven to be difficult, which is quite unfortunate because of the economic viability and scalability of such methods.
The resultant nanosheets are deficient in crystallinity, suggesting the formation of small crystalline domains with weak orientation control. These structural inadequacies hamper the performance of the nanosheet and limit researchers from exploring the nanosheet's structure-property relationships.
Recently, a group of researchers directed by Professor Hiroshi Nishihara of Tokyo University of Science (TUS) Japan, has explored whether BHT-based coordination nanosheets created by the addition of two metal ions could surpass the aforementioned challenges, in a new study, that appeared in Advanced Materials, sponsored by Japan Science and Technology Agency, Japan Society for the Promotion of Science and the White Rock Foundation.
To achieve this, the team, which also included Dr. Ryojun Toyoda and Dr. Naoya Fukui from TUS, Professor Henning Sirringhaus from the University of Cambridge, and Professor Sono Sasaki from Kyoto Institute of Technology, prepared heterometallic nanosheet films at a liquid-liquid interface, by altering the mixing ratio of two metal ions — copper (Cu) and nickel (Ni), in an aqueous solution.
In short, they poured an aqueous solution comprising these two metal ions onto an organic solution comprising a BHT precursor.
To their astonishment, they discovered that a new structural phase had developed at the interface between the two phases, with intermediate ratios of copper and nickel. Furthermore, they learned that this NiCu2BHT film comprised much higher crystallinity than pure nickel and copper films.
Dr. Nishihara and her team were particularly happy with these results because such a method typically produces nanosheets with weak crystallinity.
Our results indicate that the nanosheets grow in a specific direction and with a fixed composition, NiCu2BHT, at the liquid-liquid interface when the two metal ions are mixed at an appropriate ratio. It is extraordinary that such simple mixing of different metal ions resulted in a unique structure with 2D periodicity and enhanced crystallinity, even in relatively thick films.
Hiroshi Nishihara, Study Lead and Professor, Tokyo University of Science
Alongside a boost in crystallinity, outstanding improvements were also detected in the performance of these heterometallic nanosheets. Electrical conductivity measurements along with the examination of film morphology via electron microscopy methods showed that these films have higher conductivities and lower activation energies compared to copper films.
In fact, scientists noticed conductivities of up to 1,300 S/cm with a reliance on temperature akin to that of good metal conductors. These observations are extraordinary as such values are among the highest to be witnessed for 2D coordination nanosheets.
Finally, the team examined the fundamental mechanisms that led to this enhancement in crystalline order and proposed that NiCu2BHT films may naturally orient themselves into a bilayer structure that discharges the structural strain of the material.
It is reasonable to assume that a bilayer structure is a more favorable structural phase for heterometallic BHT-based coordination nanosheets, rather than the distorted structures of the corresponding homometallic films. Overall, our findings open a powerful new pathway to improve the crystallinity and tuning of the functional properties of highly conducting coordination nanosheets for a wide range of device applications.
Hiroshi Nishihara, Study Lead and Professor, Tokyo University of Science
It is hoped that this recent method will help scientists reap the many advantages of coordination nanosheets.
Journal Reference:
Toyoda, R., et al. (2022) Heterometallic Benzenehexathiolato Coordination Nanosheets: Periodic Structure Improves Crystallinity and Electrical Conductivity. Advanced Materials. doi.org/10.1002/adma.202106204.