It is believed that polymers with various metal complexes in their side chains will be effective high-performance materials with a broad range of applications. However, because it is difficult to regulate the metal composition, traditional fabrication techniques are not ideal for creating such polymers.
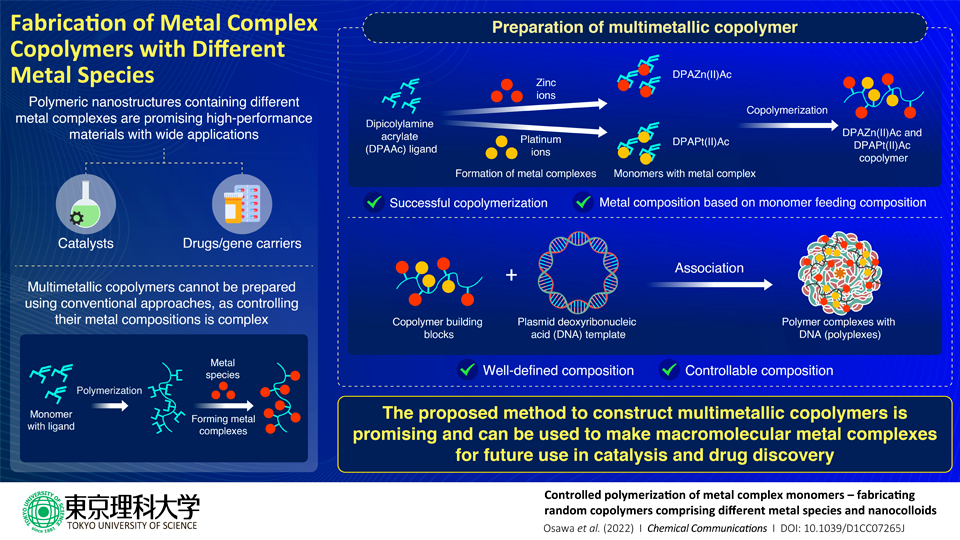
Image Credit: Tokyo University of Science
Recently, Japanese researchers found a way to get around this restriction and successfully synthesized multimetallic copolymers, which can be the basis for future hybrid materials.
Polymers are present in everything, from plastics to clothing to DNA. Long chains of the repeating units known as monomers are used to create polymers, which are extremely versatile materials. Polymers with metal complexes on their side chains offer a wide range of applications as hybrid materials. With the addition of various metal species to the polymers, this potential will only grow.
However, because it is difficult to control the composition of metal species in the final polymer, current methods of manufacturing polymers with metal complexes are unsuitable for fabricating multimetallic polymers.
A new polymerization technique that can get around this restriction has just been presented by a research team from the Tokyo University of Science, directed by Assistant Professor Shigehito Osawa and Professor Hidenori Otsuka.
The usual method of preparing such complexes is to design a polymer with ligands (molecular ‘backbones’ that join together other chemical species) and then add the metal species to form complexes on it. But each metal has a different binding affinity to the ligand, which makes it complicated to control the resulting structure.
Shigehito Osawa, Assistant Professor, Tokyo University of Science
“By considering polymerizable monomers with complexes of different metal species, we can effectively control the composition of the resulting copolymer,” added Osawa.
The work was made available online on April 1, 2022, and on April 30, 2022, it was reported in Chemical Communications, Volume 58, Issue 34.
A polymer is referred to as a copolymer when the monomers that build it up are also polymers. The researchers created the dipicolylamine acrylate (DPAAc) monomer for their research. DPA was selected because it is a superior metal-ligand and has been applied in several biological processes.
To create two polymer chains with metal complexes, DPAZn(II)Ac and DPAPt(II)Ac, they next polymerized DPAAc with zinc (Zn) and platinum (Pt). The two monomers were then copolymerized.
Researchers discovered that by adjusting the feeding content of the monomers, they could not only successfully produce a copolymer but also regulate its metal composition.
Then, using plasmid deoxyribonucleic acid (DNA) as a reference, they used this copolymer as a building component to create nanoparticles. Since the two-component monomers are proven to attach to plasmid DNA, it was selected as the template.
High-resolution scanning tunneling electron microscopy and energy-dispersive X-ray spectroscopy were used to demonstrate the formation of the resultant nanoparticle polymer complexes with DNA (polyplexes).
This process, which is now patent-pending, can be expanded to create a brand-new way to create intermetallic nanostructures.
Intermetallic catalytic nanomaterials are known to have significant advantages over nanomaterials containing only a single metallic species.
Shigehito Osawa, Assistant Professor, Tokyo University of Science
It is possible to create anti-cancer medications and gene carriers using the polyplexes created in the study because they are DNA-binding compounds. The suggested fabrication technique will also result in improvements in catalysis that shift away from platinum-based precious metals.
“These multimetallic copolymers can serve as building blocks for future macromolecular metal complexes of many varieties,” concludes Dr Osawa.
The results of this work will undoubtedly have a significant impact on polymer chemistry.
Journal Reference:
Osawa, S., et al. (2022) Controlled polymerization of metal complex monomers – fabricating random copolymers comprising different metal species and nano-colloids. Chemical Communications. doi.org/10.1039/D1CC07265J.