Magnetic materials are employed in a variety of devices, including motors, sensors, cell phones, and data storage. As technology becomes smaller and smaller, researchers are constantly hunting for new magnetic particles.
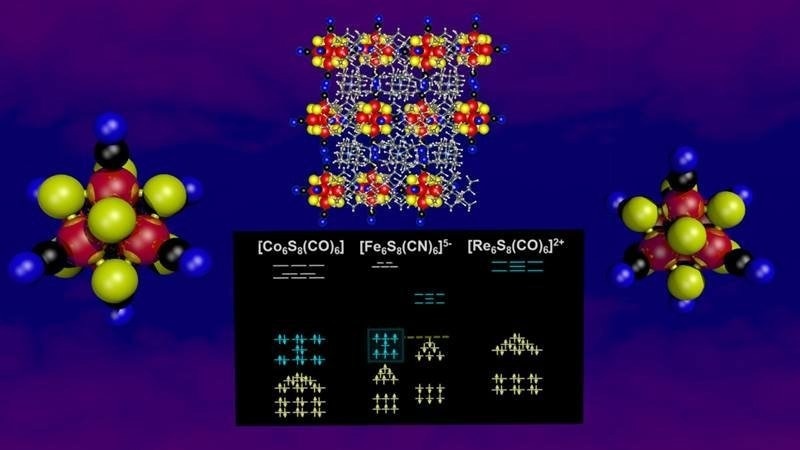 The superatom cluster (Fe6S8(CN)6-5) with a large spin magnetic moment and enhanced stability. The stability is due to dual shell filling, with the spin up and spin down shells both filled but with a different number of electrons. Image Credit: Arthur Reber, Virginia Commonwealth University
The superatom cluster (Fe6S8(CN)6-5) with a large spin magnetic moment and enhanced stability. The stability is due to dual shell filling, with the spin up and spin down shells both filled but with a different number of electrons. Image Credit: Arthur Reber, Virginia Commonwealth University
In this study, a brand-new, exceptionally small, thermally stable magnetic nanoparticle was theoretically predicted, constructed, and then produced. The nanoparticle is intended to be a small, superatom-like cluster of symmetric atoms.
Quantum confinement leads the cluster of atoms’ electronic states to take on an arrangement that resembles the electronic shells and occupancies of a single atom in superatoms.
Superatoms are endowed with unusual characteristics as a result. The new nanoparticle has two separate electronic subshells that are each packed with 57 and 50 electrons, respectively. This dual shell filling generates the energy stability required for the synthesis of superatoms as well as the stable magnetic orderings that give superatoms their special characteristics.
The Impact
The new magnetic cluster can be used to create materials with programmable features. These consist of tunable and switchable magnetic ordering, optical characteristics, and electrical conductivity. The cluster can be modified so that it can accept or give away numerous electrons. This makes multicomponent magnetic solids possible.
Also conceivable is the deposition of the cluster on two-dimensional semiconductors. Researchers can now modify semiconductors for new purposes by altering how they interact with electrons.
Summary
The classical mechanisms by which spin magnetic moments develop in atoms and ferromagnetic metals are described by Hund’s rule in atomic physics and by the Stoner model in solid-state physics. Valence electrons evade pairing in both models by occupying half-filled orbitals, which raises the exchange energy and reduces Coulomb repulsion.
Researchers from Virginia Commonwealth University, Columbia University, and Harvard University described a new mechanism in this work for the generation of magnetic moments, in which the quantum confinement of electrons in clusters separates their electronic structures into distinct subshells with different spin axes and number of available orbitals.
The cluster is stabilized, and a magnetic moment is produced by the differential spin occupations that result from each subshell being filled to attain a closed-shell electronic configuration.
This mechanism, which functions exclusively at the nanoscale, is strikingly similar to the mechanism driving magnetic semiconductors. The production of high spin states in clusters is generally prevented by Jahn-Teller distortions, but the dual subshell filling method produces great electronic stability and a significant magnetic moment.
Since the electronic structure is naturally spin-polarized and the magnetic moment produced by filling both subshells is resistant to structural distortions and flaws, this is perfect for use in spin-based electronics.
Funding
The Department of Energy Office of Science’s Basic Energy Science program provided funding for the theoretical work. The US Air Force Office of Scientific Research and the National Science Foundation’s Materials Research Science and Engineering Centers program on Precision-Assembled Quantum Materials provided funding for the experimental work.
Journal Reference:
Bista, D., et al. (2022). High-Spin Superatom Stabilized by Dual Subshell Filling. Journal of the American Chemical Society. https://pubs.acs.org/doi/10.1021/jacs.2c00731