For solar-driven H2 evolution, it is crucial to increase photocatalyst photocarrier separation efficiency and extend light absorption from the ultraviolet (UV) to the near-infrared (NIR) region.
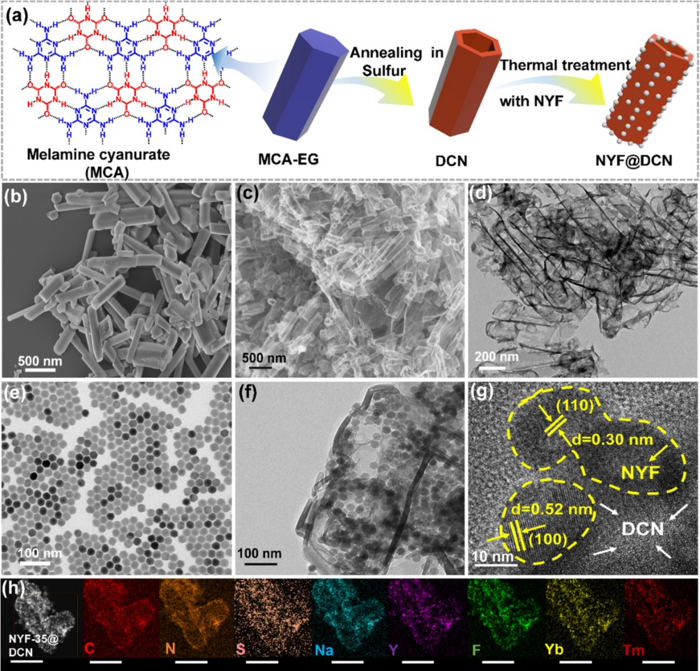 a) Schematic illustration for the fabrication of NYF@DCN. SEM images of b) precursor and c) DCN. TEM images of d) DCN, e) NYF, and f, g) NYF@DCN. h) Elemental mapping of NYF@DCN (scale bar: 500 nm). Image Credit: Science China Press
a) Schematic illustration for the fabrication of NYF@DCN. SEM images of b) precursor and c) DCN. TEM images of d) DCN, e) NYF, and f, g) NYF@DCN. h) Elemental mapping of NYF@DCN (scale bar: 500 nm). Image Credit: Science China Press
A novel interfacial regulated broadband photocatalyst made of flawed g-C3N4 (DCN) and NaYF4: Yb3+, Tm3+ (NYF) nanocrystals was recently reported in National Science Open by Prof. Gouxiu Wang’s research team from the University of Technology Sydney and Prof. Dan Wang from the Chinese Academy of Sciences (Institute of Process Engineering).
The study first authors are Prof. Nailiang Yang and Dr. Xiaochun Gao from the Institute of Process Engineering of the Chinese Academy of Sciences and the University of Technology Sydney, respectively.
By adding S dopants and C vacancies to the 3D DCN hexagonal prisms, the authors first achieved a precise defect control on g-C3N4. The broadband NYF@DCN photocatalyst was subsequently formed after the NYF nanocrystals were successfully loaded onto the DCN matrix.
Precise Interfacial Control on DCN with Optimized Defect States
The creation of S dopants and C vacancies by the use of ethylene glycol and molten sulfur was found to be essential for controlling the defect states in DCN.
The defect states can enhance the efficiency of charge separation at the interface between DCN and solution by accommodating excited electrons from the valance band and migrated electrons from the conductive band via a moderate electron-trapping ability, in addition to extending DCN’s capacity for solar absorption.
Extended Solar Harvesting of NYF@DCN
The broadband NYF@DCN showed improved solar light harvesting ability in comparison to bulk g-C3N4 (BCN) and DCN, which was primarily caused by:
1) The emergence of defect states in DCN, which reduces the excitation energy and increases the visible absorption range to 590 nm, producing a high photocurrent of 12.55 μA cm–2 under 550 nm;
2) NYF@DCN had a promoted photocurrent of 8.01 μA cm–2 as a result of the secondary excitation of DCN by the upconverted UV light from NYF crystals as reflected by the upconversion photoluminescence spectra. It is assumed that the increased solar absorption mentioned above will accelerate the solar-driven H2 evolution.
Accelerated Interfacial Charge Transfer of NYF@DCN
Theoretical calculations and the CP-MAS 13N NMR spectra revealed that, compared to BCN, a stronger interfacial charge polarization could exist via the Y-N bonding between DCN and NYF.
This is advantageous for the energy transfer from NYF to DCN via both the photo transfer (PT) pathway and the excited state energy transfer (ET) pathway.
In conclusion, the broadband NYF@DCN has a superior solar-driven H2 evolution rate of 2799 μmol h–1g–1, placing it first among g-C3N4-based photocatalysts and upconversion particle-based photocatalysts. This is due to the improved solar harvesting ability and interfacial charge transfer.
Journal Reference:
Gao, X., et al. (2022) Defect and Interface Control on Graphitic Carbon Nitrides/Upconversion Nanocrystals for Enhanced Solar Hydrogen Production. National Science Open. doi:10.1360/nso/20220037.