The pharmaceutical scientists from National University of Singapore (NUS) have come up with multi-functional synthetic peptide nanonets for alleviating inflammation resulting from bacterial infection. This is attained by simultaneous trapping of bacterial endotoxins and pro-inflammatory cytokines.
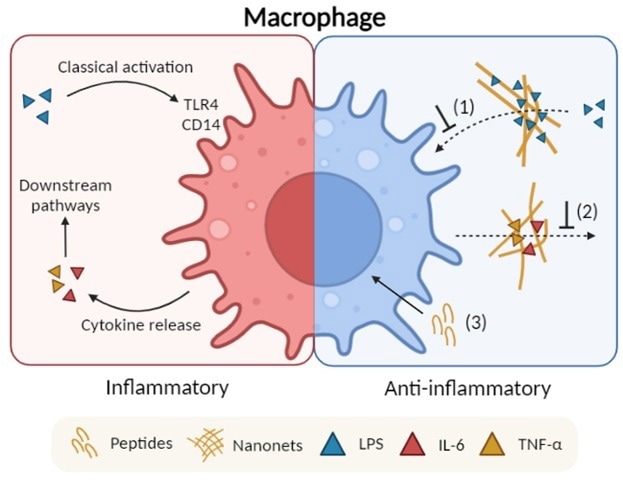
Schematic illustrates the proposed anti-inflammatory mechanisms of the fibrillating peptides: (1) Endotoxin trapping by the nanonets; (2) Cytokine trapping by the nanonets; and (3) Intracellular effect by soluble peptide molecules. Image Credit: Advanced Healthcare Materials
Endotoxemia is the existence of endotoxins in the blood. Gram-negative pathogens, like E. coli, release such endotoxins at the time of systemic infections. Inflammatory host responses can lead to extensive tissue damage and septic shock if left unchecked. These responses are linked to a high mortality rate.
In the past, previous studies have been largely unsuccessful due to the complicated nature of communications between anti- and pro-inflammatory mediators.
For better management of septic complications, a newer technique concentrates on multi-cytokine. A study group headed by Associate Professor Rachel EE from the Department of Pharmacy, NUS, established that antibacterial peptide nanonets could reduce inflammatory responses that are commonly linked to bacterial infections.
These results are developed on their past report on the design of anti-microbial peptides that have the ability of in situ self-assembly into bacteria-trapping nanonets. Anti-inflammatory activity was made possible due to the capacity of the nanonets to entrap and bind endotoxins released by gram-negative pathogens and inflammation mediators generated by host macrophages.
Of interest, the cationic nanonets entrapped pro-inflammatory cytokines selectively at the same time, slightly binding the anti-inflammatory cytokines. The researchers were successful in this by exploiting the complete difference in net charge between these two varied cytokines groups.
These results were reported in the journal Advanced Healthcare Materials.
The lipopolysaccharide (LPS)-binding effect lead to the restoration of colistin’s antimicrobial activity, a last-line therapy, against gram-negative pathogens. Remarkably, this is the first published case of multi-functional peptide nanonets that come with long-ranging effects in relieving the damages of septic complexities at several stages.
Biological examination of the peptide nanonets with the use of an acute lung injury model established their efficiency in minimizing the level of pro-inflammatory cytokines in the bronchoalveolar fluid of endotoxin-inoculated murine models. The consequent peptide effect was analogous to the control drug dexamethasone.
Prof Ee concluded, “Our peptide-based nanonets have shown unique therapeutic potential as a multi-functional biomaterial for holistic management of sepsis. Moving forward, we hope to continue their optimization for clinical use.”
Journal Reference
Tram, N. D. T., et al. (2023). Multifunctional antibacterial nanonets attenuate inflammatory responses through selective trapping of endotoxins and proinflammatory cytokines. Advanced Healthcare Materials. doi.org/10.1002/adhm.202203232.