Researchers at the University of Michigan have demonstrated how to create cage structures with nanoparticles using computer simulations, which could indicate a way to create structured nanostructures with heterogeneous materials.
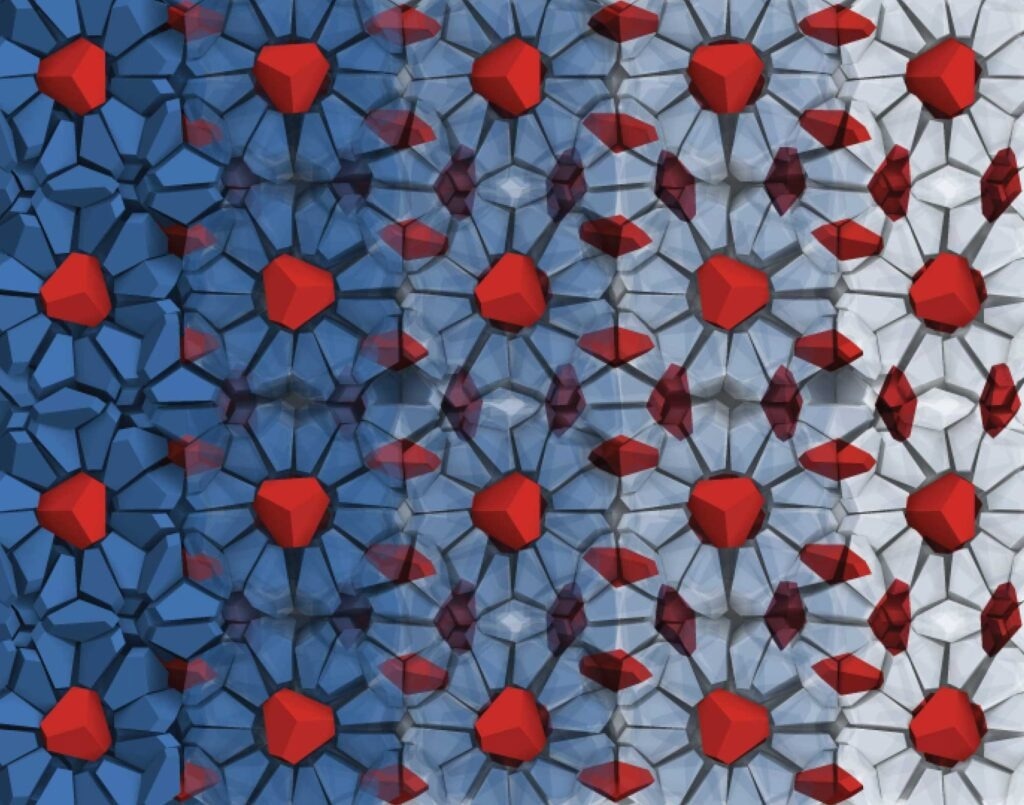 The cages of the host network of bipyramid particles are shown in blue on the left side, becoming increasingly transparent toward the right. The red bipyramid particles are guest particles, trapped in the cages of the clathrate structure. Image Credit: Sangmin Lee, Glotzer Group
The cages of the host network of bipyramid particles are shown in blue on the left side, becoming increasingly transparent toward the right. The red bipyramid particles are guest particles, trapped in the cages of the clathrate structure. Image Credit: Sangmin Lee, Glotzer Group
The discovery could provide new opportunities for photonic materials that can control light differently than natural crystals. Additionally, it demonstrated a peculiar outcome that the group called entropy compartmentalization.
We are developing new ways to structure matter across scales, discovering the possibilities and what forces we can use. Entropic forces can stabilize even more complex crystals than we thought.
Sharon Glotzer, Study Lead, Anthony C. Lembke Department Chair, Chemical Engineering, University of Michigan
The tendency of a system to maximize its conceivable states is more appropriately reflected by entropy, which is sometimes characterized as a system’s disorder.
This frequently results in disarray in the normal sense. Instead of clustering in a corner, oxygen molecules fanned out to occupy the space. The correct size box, however, will cause them to automatically arrange themselves into a recognizable form.
The same applies to nanoparticles. Glotzer’s team has previously demonstrated that bipyramid particles, two tiny, three-sided pyramids attached at their bases, would form formations mimicking those of fire ice if placed in sufficiently small boxes.
Water molecules that surround methane to create cages make up fire ice, which can melt and burn simultaneously. This material is an example of a clathrate and is widely distributed beneath the ocean floor.
Clathrate structures are being researched for a variety of uses, including the collection and removal of carbon dioxide from the environment.
Earlier nanoparticle clathrate structures, unlike water clathrates, did not have gaps to be filled with other materials that would present new and intriguing opportunities for changing the structure’s characteristics. The group sought to alter that.
This time, we investigated what happens if we change the shape of the particle. We reasoned that if we truncate the particle a little, it would create space in the cage made by the bipyramid particles.
Sangmin Lee, Study First Author, Postdoctoral Student, University of Michigan
He removed the three middle corners from each bipyramid and found the sweet spot where openings occurred in the structure, but the sides were still intact enough to prevent the pyramids from reorganizing.
When they were the only particle present in the system, the voids were filled by additional truncated bipyramids. The form that was inserted as a second shape eventually evolved into the trapped guest particle.
There was no glue keeping the bipyramids together in this instance, despite the fact that Glotzer has ideas on how to make sides that are selectively adhesive so that various materials can serve as cages and guest particles. Instead, entropy completely maintained the structure.
Glotzer added, “What is really fascinating, looking at the simulations, is that the host network is almost frozen. The host particles move, but they all move together like a single, rigid object, which is exactly what happens with water clathrates. But the guest particles are spinning around like crazy—like the system dumped all the entropy into the guest particles.”
This was the system that the truncated bipyramids could construct in the smallest amount of space, yet almost all of the flexibility belonged to the guest particles. According to the researchers, methane also spins in water clathrates.
Furthermore, when the guest particles were taken out, the structure hurled bipyramids that had been a part of the networked cage structure into the interiors of the cages since it was more crucial to have spinning particles accessible than full cages to increase entropy.
Glotzer further stated, “Entropy compartmentalization. Isn’t that cool? I bet that happens in other systems too—not just clathrates.”
The study was assisted by Thi Vo, who is currently an assistant professor of chemical and biomolecular engineering at Johns Hopkins University and was formerly a postdoctoral researcher in chemical engineering at the University of Michigan.
The Extreme Science and Engineering Discovery Environment of the National Science Foundation and the University of Michigan supplied the computing resources for this study, which was supported by the Department of Energy and Office of Naval Research.
Glotzer is also a materials science and engineering, macromolecular science and engineering, and physics professor, as well as the John Werner Cahn Distinguished University Professor of Engineering and the Stuart W. Churchill Collegiate Professor of Chemical Engineering.
Journal Reference:
Lee, S., et al. (2023) Entropy compartmentalization stabilizes open host–guest colloidal clathrates. Nature Chemistry. doi:10.1038/s41557-023-01200-6.