This study was collaboratively performed by Dr. Shu-Hong Yu, Dr. Long-Ping Wen, and Dr. Kun Qu from the University of Science and Technology of China, with Professor Yang Lu from the Hefei University of Technology.
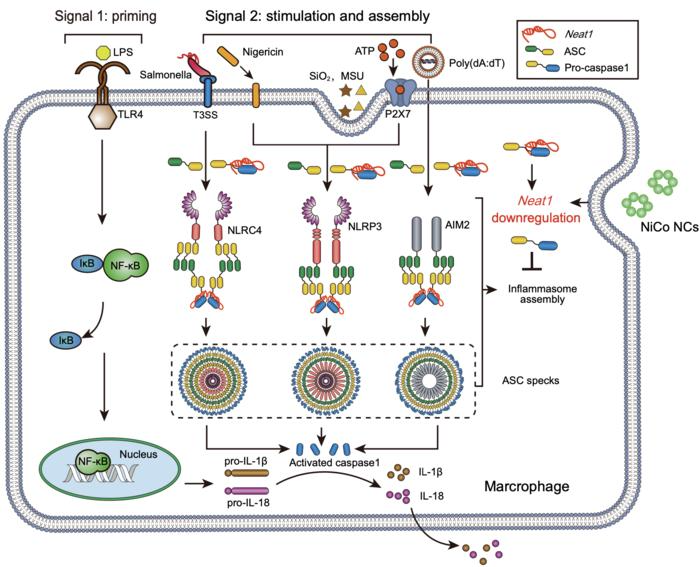
Multiple stimuli trigger the activation of three inflammasomes: NLRP3, NLRC4, and AIM2. NiCo NCs suppressed the inflammasome assembly process by downregulating the long non-coding RNA Neat1, thereby inhibiting the activation of three inflammasomes. Image Credit: ©Science China Press
Too much activation of inflammasomes has been linked to several diseases, such as atherosclerosis, Alzheimer’s disease, gout, and type 2 diabetes.
Provided the pivotal role of macrophages in both nanoparticle phagocytosis and inflammasome activation, the breakthrough of anti-inflammatory nanoparticles, particularly targeting macrophages, could more efficiently modulate inflammatory response while reducing off-target effects in other cell kinds.
But most presently reported nanomaterials have been discovered to encourage instead of hinder inflammasome activation.
The study showed that nickel-cobalt alloy nanocrystals display outstanding efficacy in suppressing the activation of three inflammasomes in main macrophages, namely NLRP3, AIM2, and NLRC4.
Later, the scientists employed two disease models, colitis and acute peritonitis, to assess the effect of nickel-cobalt alloy nanocrystals on treating inflammasome overactivation.
The outcomes showed how nickel-cobalt alloy nanocrystals efficiently ameliorated disease symptoms in mice in the colitis model, such as restoring colon length, mitigating weight loss, and mitigating damage to the intestinal mucosal epithelium. Moreover, in the acute peritonitis model, such nanocrystals considerably attenuated neutrophil chemotaxis inside the peritoneal cavity of mice.
To verify if nickel-cobalt alloy nanocrystals need cellular internalization to apply their anti-inflammatory effects, the authors performed experiments with the help of an extensively utilized endocytosis inhibitor named cytochalasin D.
Treatment performed with cytochalasin D considerably decreased the internalization of nickel-cobalt alloy nanocrystals by macrophages. Moreover, hindering the internalization of the nanocrystals by macrophages has resulted in a decrease in their anti-inflammatory effects. This shows that the anti-inflammatory action of nickel-cobalt alloy nanocrystals depends on their cellular uptake.
To analyze if the anti-inflammatory impacts of nickel-cobalt alloy nanocrystals are assigned to their elemental composition or geometric morphology, the authors synthesized cobalt nanoparticles and nickel nanoparticles under identical conditions as controls. This displayed diverse morphologies in comparison to the nickel-cobalt alloy nanocrystals.
But both cobalt nanoparticles and nickel nanoparticles also considerably restrained inflammasome activation.
Hence, the authors assigned the inhibitory effect of nickel-cobalt alloy nanocrystals to the elemental composition instead of their geometric shape. Such findings denote that nanomaterials consisting of cobalt and nickel might provide opportunities for developing nano-drugs with anti-inflammatory properties.
Disclosing the biological mechanisms underlying the action of nanomaterials is considered vital for their possible medical applications. But clarifying the biological mechanism by which such broad-spectrum anti-inflammatory nanocrystals curb inflammasome activation poses considerable difficulties using traditional biological experimental approaches.
To fulfill this, scientists performed RNA sequencing and the assay for transposase-accessible chromatin with sequencing (ATAC-Seq). This resulted in the identification of an earlier reported non-coding RNA, Neat1, considered to be involved in inflammasome assembly.
After treatment with the nickel-cobalt alloy nanocrystals, the expression of Neat1 was considerably decreased. Earlier studies have illustrated that downregulating Neat1 expression alone significantly prevents the activation of NLRC4, NLRP3, and AIM2 inflammasomes.
ATAC-Seq outcomes disclosed a considerable decrease in the chromatin accessibility of the gene body and promoter regions of Neat1 in the nickel-cobalt alloy nanocrystal-treated group. This denotes that the inhibition of inflammasome activation by the nickel-cobalt alloy nanocrystals has been obtained by suppressing Neat1 transcription instead of fostering its degradation.
Journal Reference
Lin, J., et al. (2023) Nickle-cobalt alloy nanocrystals inhibit activation of inflammasomes. National Science Review. doi.org/10.1093/nsr/nwad179.