The University of California San Diego’s engineers have created modular nanoparticles that are easily customizable to target viruses, cancers, or toxins. As the nanoparticles’ surface is designed to accommodate any desired biological molecule, they can be customized for a variety of uses, such as neutralizing biological agents or delivering drugs on a targeted basis.
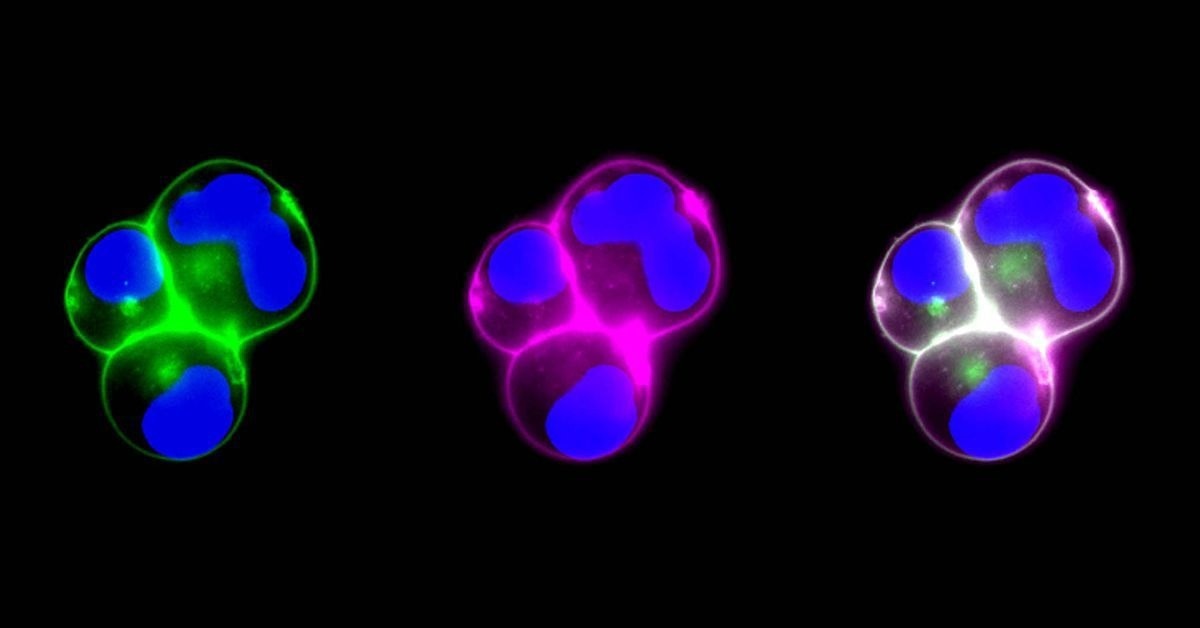 Live cell fluorescent visualization of biological molecules binding to the surface of genetically modified cell membranes, which serve as the coating for the modular nanoparticles. Image Credit: University of California, San Diego
Live cell fluorescent visualization of biological molecules binding to the surface of genetically modified cell membranes, which serve as the coating for the modular nanoparticles. Image Credit: University of California, San Diego
This technology’s efficiency and simplicity are what make it so appealing. Rather than creating unique nanoparticles for every use case, scientists can now employ a modular nanoparticle base to easily affix proteins that are intended to target a particular biological target.
Previous methods involved going through a new synthetic process from beginning to end to create unique nanoparticles for various biological objectives. However, using this novel method, a complete range of specialized nanoparticles can be easily created by modifying the same modular nanoparticle base.
This is a plug and play platform technology that allows for rapid modification of a functional biological nanoparticle.
Liangfang Zhang, Professor, Nanoengineering. Jacobs School of Engineering, University of California, San Diego
A study by Zhang and his team describing their research was published in Nature Nanotechnology on October 30th, 2023.
The genetically engineered cell membranes are coated on biodegradable polymer cores to create modular nanoparticles. The secret to their modular structure is a pair of artificial proteins called SpyCatcher and SpyTag, which are engineered to connect exclusively and spontaneously.
In biological research, this pair is frequently utilized to combine different proteins. In this study, Zhang and his team used the pair to easily design a method for binding proteins of interest to the surface of a nanoparticle.
This is how it operates: SpyTag is chemically bound to a protein of interest, such as a protein that targets viruses or cancers, whereas SpyCatcher is attached to the surface of the nanoparticle. SpyTag-linked proteins bond easily to SpyCatcher-decorated nanoparticles when they come into touch with one another, making it possible for target proteins to be easily attached to the surface of the nanoparticle.
For instance, SpyTag can be connected to a protein that finds tumor cells to target tumors; the protein that is then linked to SpyTag is affixed to the nanoparticle. The procedure is equally simple if the target changes to a particular virus: attach SpyTag on the surface of the nanoparticle and link it to a protein that targets the virus.
It is a very simple, streamlined and straightforward approach to functionalizing nanoparticles for any biological application.
Liangfang Zhang, Professor, Nanoengineering. Jacobs School of Engineering, University of California, San Diego
Human embryonic kidney (HEK) 293 cells, a frequently used cell line in biological research, were first genetically altered to generate SpyCatcher proteins on their surface so that the researchers could create the modular nanoparticles. After separation and fragmentation, the cell membranes were deposited onto biodegradable polymer nanoparticles.
Following that, the nanoparticles were combined with SpyTag-linked proteins. The researchers utilized two separate proteins in this study: one that targeted the epidermal growth factor receptor (EGFR) and the other that targeted the human epidermal growth factor receptor 2 (HER2), both of which are found on the surface of cancer cells.
The nanoparticles were evaluated in mice with ovarian cancers as a proof of concept. Docetaxel, a chemotherapy medication, was put into the nanoparticles and administered to mice via intravenous injection every three days for a total of four injections. Treatment with these nanoparticles inhibited tumor development while increasing survival. Untreated mice had a median survival of 24 to 29 days, whereas treated mice had a median survival of 63 to 71 days.
The researchers are working to develop a modular nanoparticle platform for specific drug delivery.
Zhang is enthused about the possible applications of this technique beyond cancer treatment.
Zhang noted, “Because we have a modular nanoparticle base, we can easily attach a neutralizing agent on the surface to neutralize viruses and biological toxins. There is also potential for creating vaccines by attaching an antigen on the nanoparticle surface using this modular platform. This opens the door to a variety of new therapeutic approaches.”
The National Institutes of Health (R01CA200574, R21AI159492, and R21AI175904), the National Science Foundation (DMR-1904702), and the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense (HDTRA1-21-1-0010) provided funding for this work.
Journal Reference:
Krishnan, N., et al. (2023) A modular approach to enhancing cell membrane-coated nanoparticle functionality using genetic engineering. Nature Nanotechnology. doi:10.1038/s41565-023-01533-w