Human noroviruses cause acute gastroenteritis, a worldwide health issue for which no vaccines or antiviral drugs are available. Although most healthy individuals recover entirely, norovirus can be fatal in newborns, the elderly, and persons with underlying conditions. Human noroviruses are estimated to cause 684 million infections and 212,000 deaths per year.
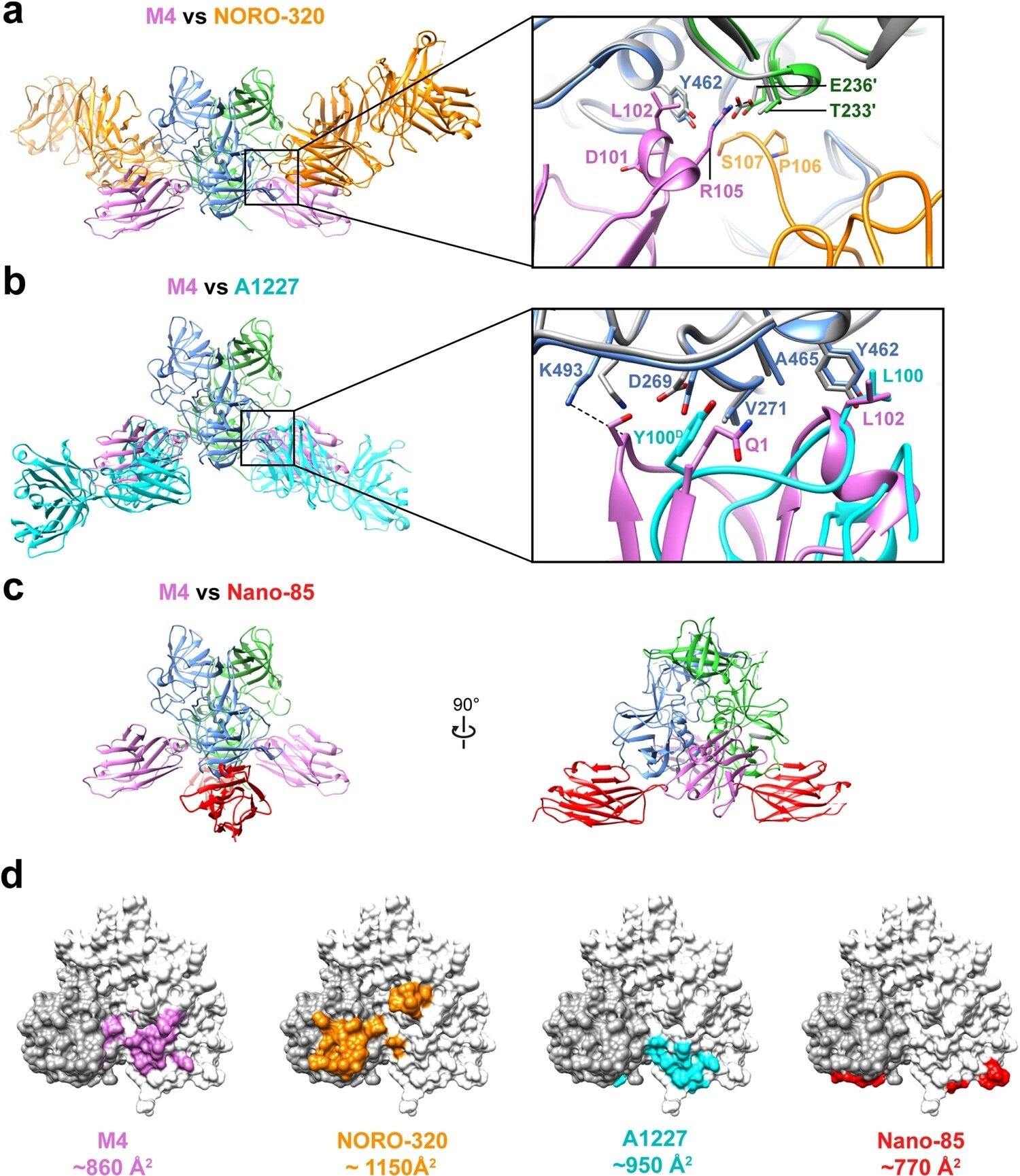 Comparison of M4-GII.4 P-domain complex with representative GII.4-mAb or GII.4-nanobody complexes. a–c Superimposition of the structure GII.4 P-domain in complex with M4 (pink, PDB ID: 8G0W), NORO-320 Fab (orange, PDB ID: 7JIE), A1227 Fab (cyan, PDB ID: 6N81), or nanobody Nano-85 (red, PDB ID: 4X7D). The subunits A or B of the P-domain dimer are colored in blue and green, respectively. The insets in (a) and (b) show the close-up view of the epitopes on the P-domain, with key side chains shown in the stick model and labeled. d The P-domain dimers are shown with white and dark gray surface representation to define each subunit, with the buried surface on P-domain colored as the corresponding nanobody or Fab. Image Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-42146-0
Comparison of M4-GII.4 P-domain complex with representative GII.4-mAb or GII.4-nanobody complexes. a–c Superimposition of the structure GII.4 P-domain in complex with M4 (pink, PDB ID: 8G0W), NORO-320 Fab (orange, PDB ID: 7JIE), A1227 Fab (cyan, PDB ID: 6N81), or nanobody Nano-85 (red, PDB ID: 4X7D). The subunits A or B of the P-domain dimer are colored in blue and green, respectively. The insets in (a) and (b) show the close-up view of the epitopes on the P-domain, with key side chains shown in the stick model and labeled. d The P-domain dimers are shown with white and dark gray surface representation to define each subunit, with the buried surface on P-domain colored as the corresponding nanobody or Fab. Image Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-42146-0
Human noroviruses are highly diverse. Noroviruses are categorized into ten groups, of which groups GI, GII, GIV, GVIII, and GIX infect humans. Viruses in the GII.4 subgroup are the most predominant in human populations.
Dr. Wilhelm Salmen, Postdoctoral Fellow, University of Michigan
Noroviruses are also infamous for producing new varieties on a regular basis, notably those of GII.4 norovirus, which can circumvent the immune response generated by the body against prior versions, as can several flu viruses and coronaviruses.
The diversity of norovirus groups, as well as the recurrence of new variations, are among the variables posing challenges to the development of efficient preventative and therapeutic measures to manage this devastating disease.
Salmen, Prasad, and their colleagues studied a unique technique to kill human noroviruses in their latest study, which was published in the journal Nature Communications. They investigated if nanobodies, or microscopic antibodies generated by llamas, might efficiently counteract human norovirus infection in the lab.
The surprising findings suggest that nanobodies might be used to build a therapeutic treatment against human norovirus.
Llama Nanobodies May Give an Upper Hand
Llamas and similar species, such as camels and alpacas, create antibodies for disease defense in the same way as humans do. Llamas’ antibodies, on the other hand, are roughly a twentieth the size of human antibodies. Llama's nanobodies were created to combat viruses that cause hepatitis B, influenza, human immunodeficiency, polio, and other diseases.
“Our collaborators from Argentina, Dr. Marina Bok and Dr. Viviana Parreño at the Institute of Virology and Technology Innovation, had prepared nanobodies from llamas that were inoculated with human norovirus-like particles from different strains. We worked with one nanobody named M4, which bound to the predominant GII.4 strain, testing its ability to neutralize different norovirus strains, that is, to prevent them from infecting human cells,” Dr. Salman added.
The nanobodies were examined for their capacity to prevent live viruses from invading human intestinal organoids or small intestines made in the lab. Mini guts are human intestinal cell models that closely resemble genuine small intestine tissue and its activities, allowing scientists to examine how noroviruses behave and test possible cures.
It was really unexpected to see that the M4 nanobody not only interacted and neutralized the currently circulating pandemic GII.4 strain but also its older variants.
Dr. B V Venkataram Prasad, Alvin Romansky Chair in Biochemistry and Professor, Verna and Marrs McLean Department of Biochemistry, Baylor College of Medicine
In addition, he is the study’s corresponding author and a member of Baylor’s Dan L. Duncan Comprehensive Cancer Center.
While they expected the M4 nanobody to recognize only the GII.4 strain used to generate M4, the researchers employed crystallography and other techniques to closely examine the interactions between nanobodies and noroviruses in an attempt to understand how the M4 nanobody recognizes and neutralizes a variety of noroviruses.
Salmen added, “We discovered that this little nanobody can recognize a part of the norovirus that all the different noroviruses that we tested have in common.”
Norovirus Particles: A Dynamic Structure
The researchers found that the M4 nanobody identified a concealed compartment inside the norovirus particles, which would only become visible if the particles experienced a structural alteration.
“The traditional thinking is that viral particles are in a very stable compact state, but in reality, these particles 'breathe' considerably. Recent studies have shown that the structure of norovirus particles is dynamic, alternating between a resting or compact conformation and a raised conformation,” Salmen stated.
Prasad noted, “We think that the raised state is important for the virus to bind to cells and infect them. We also think that when the viral particles are in the raised state, the hidden pocket is exposed and available for the nanobody to bind to it and, acting like a wedge, to keep the particle in an elevated, potentially unstable state, preventing it from collapsing back down into the compact, more stable resting state.”
Salmen further added, “Our findings suggest that trapping the viral particles in an elevated, unstable state disassembles the particles, which kills the virus. This effectively would stop the infection as it blocks the transmission chain, preventing the virus from spreading from cell to cell.”
Dr. Mary Estes, Study Co-Author and Distinguished Service Professor of Virology and Microbiology and Cullen Foundation Endowed Chair at Baylor, added, “This study is also remarkable in confirming that the human norovirus must change its 3D confirmation, from compact to raised, to infect people.”
She is also a part of the Dan L. Duncan Comprehensive Cancer Center at Baylor.
“Also, this work reveals the importance of considering viral particle dynamics when designing vaccines,” she concluded.
Journal Reference:
Salmen, W. et al. (2023) A single nanobody neutralizes multiple epochally evolving human noroviruses by modulating capsid plasticity. Nature Communications. doi:10.1038/s41467-023-42146-0