Dr. Yu Zheng, together with her graduate student Qin Qin (from the Department of Biotherapy, Cancer Center and State Key Laboratory of Biotherapy, West China Hospital, Sichuan University), developed and wrote the review.
 Nanoparticles have been placed great expectations for inflammation targeting therapy. This review mainly elaborates on action mechanism of inflammation-targeting vectors, nanoparticle classification based on targeting moiety category, methods to combine targeting moiety with core nanoparticles, techniques for assessing targetability in vitro and in vivo and finally the challenges and prospects in this area, which would provide specific and practical guidance for researchers to construct and evaluate inflammation targeting vectors rationally. Image Credit: Sichuan International Medical Exchange & Promotion Association
Nanoparticles have been placed great expectations for inflammation targeting therapy. This review mainly elaborates on action mechanism of inflammation-targeting vectors, nanoparticle classification based on targeting moiety category, methods to combine targeting moiety with core nanoparticles, techniques for assessing targetability in vitro and in vivo and finally the challenges and prospects in this area, which would provide specific and practical guidance for researchers to construct and evaluate inflammation targeting vectors rationally. Image Credit: Sichuan International Medical Exchange & Promotion Association
The physiological or pathological process of inflammation occurs when the body attempts to reestablish internal homeostasis in response to stimuli, including infection, tissue stress, malfunction, or damage, as well as physical stimulation provocation such as hypoxia. Additionally, it is linked to the onset and progression of many chronic and acute diseases, such as pancreatitis, rheumatoid arthritis, sepsis, atherosclerosis, ischemic heart and brain disease, etc.
Inflammatory cells are attracted to the lesion and execute numerous effector roles to eradicate infection or repair harm throughout this phase. A moderate inflammatory response is advantageous. Excessive inflammatory response can result in severe pathological syndromes such as systemic inflammatory response syndrome (SIRS), cytokine storm (CS), multiple organ failure (MOF), and even death.
Traditional anti-inflammatory drugs, such as steroids, non-steroids, anti-leukotrienes, pro-inflammatory cytokine inhibitors, anti-inflammatory peptides, and small interfering RNAs (siRNA), have drawbacks such as non-specific tissue distribution, low bioavailability, and a short half-life, resulting in off-target side effects and limited disease control efficacy.
Nanoparticles have developed as a unique treatment paradigm in this field to address these challenges. These drug-delivery vectors targeting inflammation primarily use the difference in the inflammatory milieu between inflamed and normal tissue to accomplish vector enrichment and sequestration.
Vascular response and leukopedesis are the main components of the early inflammatory phase. Several components, such as selectins, integrins, cellular adhesion molecules (CAMs), and others, involved in leukocyte adherence, rolling, and capture have been extensively studied to expand vector targeting. Only carriers possessing pleiotropic qualities will have favorable application possibilities, given that inflammation is a multi-signal, intricate process.
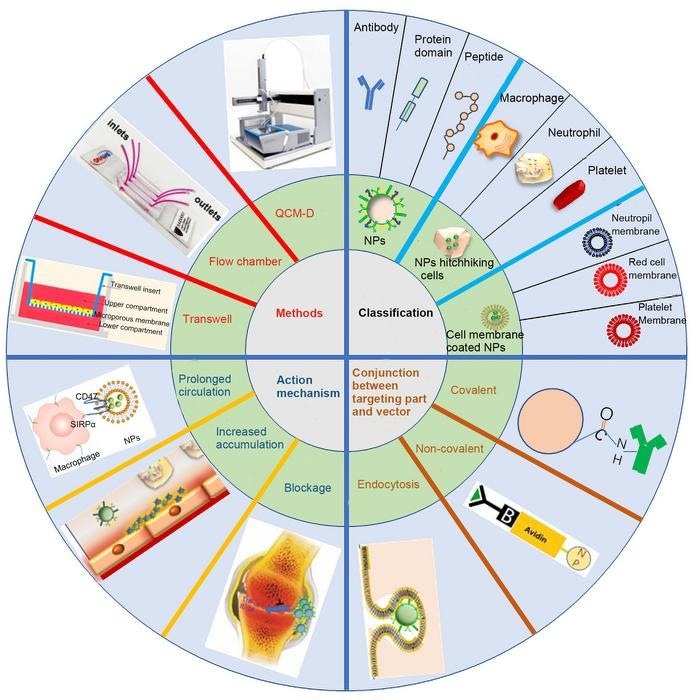
It is necessary to be familiar with the common action mechanism of current carriers to build future carriers in a more acceptable manner. An extensive summary of the mechanism of action of inflammation-oriented vectors was provided by Qin and Yu.
This mechanism includes blocking the progression of inflammation by inflammatory mediators, increasing the accumulation of nanoparticles at inflamed tissues by using inflammatory signals, and prolonging systemic circulation by inhibiting systemic clearance.
This review also presents strategies to combine targeting moiety with core nanoparticles, methods to classify nanoparticles based on targeting moiety category, and approaches to assess targetability in vitro and in vivo, to give researchers targeted and useful guidance for the rational construction and evaluation of inflammation targeting vectors.
Active targeting and passive targeting are the two types of drug delivery methods that target inflammation. Albumin-based nanoparticles and lipoprotein mimetic nanotherapeutics were the passive targeting vectors; they were recognized by their natural receptors expressed in phagocytes.
Different targeting modalities, such as imitating the interaction region with a protein, protein domain, or peptide, using antibodies for recognition, hiding the cellular membrane, using natural ligands of inflammatory receptors, or using nanoparticles as hitchhikers on inflammatory cells, can be used to achieve the active targeting effect of the vectors.
The vector was modified using ligands through the use of both covalent and non-covalent coupling. Electrostatic adsorption and covalent coupling were often used to associate the virus with inflammatory cells. Furthermore, endocytosis of the vector was frequently employed to produce inflammatory cells akin to Trojan horses.
When it comes to prescription optimization—which is quicker, more affordable, and simpler in batch screening—targeting assessment in vitro is always significant. Related techniques include surface plasmon resonance, transwell test, dynamic flow chamber, internalization of the nanoparticles by inflammatory cells, quartz crystal microbalance with dissipation monitoring, etc.
Even though highly skilled in vitro assays aim to mimic the physiological or pathological microenvironment, the intricate internal milieu, and dynamic change characteristics during an inflammatory state necessitate the use of an animal model in an in vivo assay to accurately assess the performance of vectors.
Using a variety of modern techniques, including fluorescence imaging, magnetic resonance imaging, and radionuclide scintigraphy, the spatiotemporal biodistribution of the vectors was assessed both qualitatively and quantitatively following administration. This provided a useful foundation for additional vector optimization.
Most studies on drug-loaded nanoparticles for the treatment of inflammatory diseases have barely reached the preclinical stage, despite tremendous advancements in nanotechnology over the last ten years. No biomimetic nanoparticle targeting inflammation has advanced to the clinical stage of research as of yet.
It is highly anticipated that novel nanotechnology platforms, the completion of the safety assessment system for nanocarriers, and enhanced pilot production will lead to the eventual clinical study of nanoparticle drug delivery systems for anti-inflammatory drugs, thereby ushering in a new era in the diagnosis and treatment of inflammation.
Journal Reference:
Qin, Q., et. al. (2023) Development of nanoparticle-based drug delivery system for inflammation treatment and diagnosis. MedComm – Biomaterials and Applications. doi:10.1002/mba2.65