Amino acids, such as tyrosine and tryptophan, are the essential building blocks of proteins. Since these biomolecules have distinct chemical groups on each end and side chain, they have the inherent potential to form a chain by creating an amide (peptide) bond. However, such connections are weak and readily damaged under physiological settings. This is the point where the Fmoc-protected amino acids come into play.
 Nanotechnology confers the ability to tailor the surface properties of nanoparticles and enhance the overall drug release profile. The use of tannic acid coating along with loading of the anticancer drug – doxorubicin, creates a smart drug delivery system that can be triggered to release the drug in a controlled and sustained manner, resulting in increased anticancer efficacy. Image Credit: Eijiro Miyako from JAIST.
Nanotechnology confers the ability to tailor the surface properties of nanoparticles and enhance the overall drug release profile. The use of tannic acid coating along with loading of the anticancer drug – doxorubicin, creates a smart drug delivery system that can be triggered to release the drug in a controlled and sustained manner, resulting in increased anticancer efficacy. Image Credit: Eijiro Miyako from JAIST.
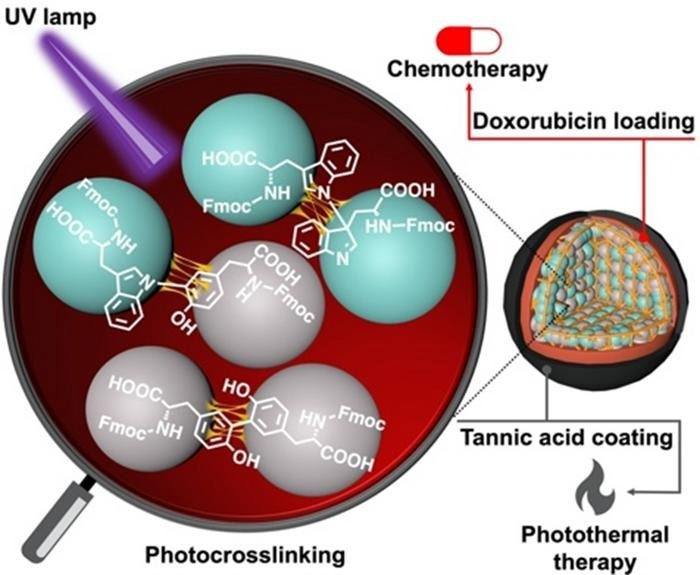
The Fmoc-protected amino acids were crosslinked using ultraviolet light at 254 nm (resulting in CBPUV nanoparticles) and riboflavin-mediated crosslinking at 365 nm (resulting in CBPRibo nanoparticles) by a research team headed by Dr. Eijiro Miyako, Associate Professor, Japan Advanced Institute of Science and Technology (JAIST), as well as Dr Alberto Bianco and Dr Cécilia Ménard-Moyon from the Centre National de la Recherche Scientifique (CNRS), France.
Amino acids being the building blocks of proteins have numerous advantages, such as better biocompatibility. Therefore, we wanted to create novel self-assembled amino acid-based nanoparticles which can be triggered through multiple mechanisms.
Dr. Eijiro Miyako, Associate Professor, Japan Advanced Institute of Science and Technology
The study’s conclusions are published in Small.
The stably crosslinked dimers of Fmoc-Tyr-OH (tyrosine) and Fmoc-Trp-OH (tryptophan) comprised the self-assembled amino acids. The crosslinked amino acid nanoparticles were then filled with the anticancer medication doxorubicin. Tannic acid-Iron (Fe3+) combination, or TAF, was employed by the researchers as the outer layer of coating to improve the stability of the nanoparticles.
This coating could break down inside the cells due to a pH differential in the tumor microenvironment or the enzymatic release of glutathione. In photothermal anticancer treatment, the tannic acid coating can raise the local temperature around the cancer tissue, killing cancer cells.
After that, a thorough investigation was conducted on the produced nanoparticles to determine their stability, drug release, and structural integrity at various pH levels. Next, employing methodologies from cell culture, the functional profile, cellular absorption, and biocompatibility of self-assembled amino acid nanoparticles were investigated.
Lastly, tumor-bearing mice were used to assess the anticancer activity of synthetic nanoparticles. The combination of photothermal treatment (facilitated by the tannic acid coating) and chemotherapy (due to the doxorubicin effect) demonstrated remarkable anticancer efficacy.
The amino acid-based nanoparticles’ size, color, absorbance, fluorescence, and thermal stability changed significantly after crosslinking. In addition, CBPUV showed better stability than CBPRibo upon crosslinking. Moreover, CBPUV continuously preserved its structure, while CBPRibo displayed partial disintegration that resulted in hollow spheres.
Drug release research conducted at physiological pH (7.4) showed negligible drug release, suggesting that persistent coating is essential for in vivo administration. The drug release from the coating was minimal at pH 5.5 due to incomplete breakdown.
However, GSH/pH responsiveness was demonstrated by the addition of glutathione (GSH) at pH 5.5, which dramatically increased drug release by inducing TAF coating breakdown. Coating breakdown was accelerated by the combination of acidic and GSH treatment.
Under some physiological circumstances, this responsive response allows for regulated medication release. Moreover, concentration-dependent cytotoxicity and enhanced effectiveness in combination chemotherapy and photothermal treatment were demonstrated by in vitro evaluations. Significant tumor growth suppression was shown in vivo experiments on tumor-bearing mice, suggesting promising anticancer actions without discernible adverse effects.
Dr. Miyako added, “Nanotechnology holds the promise of transforming basic laboratory science into a powerful tool for combating complex diseases like cancer. We are optimistic that this pioneering research will advance, potentially evolving into cutting-edge cancer treatment technology ready for clinical trials within ten years.”
In the future, the creation of these self-assembling amino acid nanoparticles could help in combating important problems like cancer’s multidrug resistance and enhance the general effectiveness of treatment results.
Journal Reference:
Wang, T., et. al. (2023) Photocrosslinked Co-Assembled Amino Acid Nanoparticles for Controlled Chemo/Photothermal Combined Anticancer Therapy. Small. doi:10.1002/smll.202307337