Sep 18 2007
Researchers at the Georgia Institute of Technology have developed a miniature sensor that uses polymer membranes deposited on a tiny silicon disk to measure pollutants present in aqueous or gaseous environments. An array of these sensors with different surface coatings could be used during field-testing to rapidly detect many different chemicals.
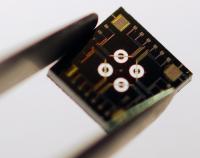 Photograph of the microsensor chip with four disk-type microresonators in the center. The size of the chip is 3.5 millimeters by 3.5 millimeters.
Photograph of the microsensor chip with four disk-type microresonators in the center. The size of the chip is 3.5 millimeters by 3.5 millimeters.
Since this new sensor allows water and air samples to be analyzed in the field, it is an improvement over classical techniques that require samples be carried back to the laboratory for analysis. This research, funded by the National Science Foundation, was presented on August 20 at the American Chemical Society’s 234th National Meeting.
The heart of the disk-shaped sensor is a microbalance that measures the mass of pollutant molecules.
“When pollutant chemicals get adsorbed to the surface of the sensor, a frequency change of the vibrating microbalance provides a measure of the associated mass change,” said Oliver Brand, associate professor in Georgia Tech’s School of Electrical and Computer Engineering.
Cantilever-type balances, which move up and down like a diving board, are common when measuring the amount of a chemical in the gas phase. However, the mechanical vibrations of the balance used to detect the mass changes are damped in liquids, causing the sensitivity of the balance to decrease. Thus, Brand and graduate students Jae Hyeong Seo, Stuart Truax and Kemal Safak Demirci searched for structures whose vibrations were less affected by the surrounding medium.
The researchers chose a silicon disk platform for the sensor. The disk shears back and forth around its center with a characteristic resonance frequency between 300 and 1,000 kHz, depending on its geometry. With proper actuation and sensing elements integrated onto the microstructures, Brand can electrically excite the resonator and sense these rotational oscillations.
Since each sensor has a diameter of approximately 200-300 microns, or the average diameter of a human hair, an array of a dozen sensors is only a few millimeters in size.
To determine how to selectively detect multiple pollutants in the same sample, Brand began collaborating with Boris Mizaikoff, an associate professor in Georgia Tech’s School of Chemistry and Biochemistry and director of its Applied Sensors Laboratory.
Mizaikoff and graduate students Gary Dobbs and Yuliya Luzinova selected commercially available hydrophobic polymers and deposited them as thin film membranes on the sensor surface. They continue to investigate innovative ways to consistently deposit the polymers at the disk surface, while ensuring sufficient adhesion for long-term field applications.
“By modifying the silicon transducer surface with different polymer membranes, each sensor becomes selective for groups of chemicals,” explained Mizaikoff.
An array of these sensors, each sensor with a different chemically modified transducer surface, can sense different pollutants in a variety of environments ranging from industrial to environmental and biomedical monitoring applications.
Brand and Mizaikoff aim to detect volatile organic compounds (VOCs) in aqueous and gaseous environments. VOCs are pollutants of high prevalence in the air and surface and ground waters. They are emitted from products such as paints, cleaning supplies, pesticides, building materials and furnishings, office equipment and craft materials.
A common VOC is benzene, with a maximum contaminant level set by the Environmental Protection Agency (EPA) at five micrograms per liter in drinking water. Many VOCs are present at similar very low concentrations, so effective sensors must accurately measure and discriminate very small mass changes.
“We’ve been able to measure concentrations among the lowest levels that have been achieved using this type of resonant microsensor,” noted Brand. “While we have not achieved the required sensitivity yet, we are constantly making improvements.”
Brand and Mizaikoff have tested their sensor device in the laboratory by pumping water with specific pollutant concentrations through a simple flow cell device attached to the sensor.
A typical test begins by flowing a water sample containing a known amount of pollutant over a sensor coated with a polymer membrane. When the sample flows through the cell, the mass of the microstructure increases, causing its characteristic vibration frequency, or resonance frequency, to decrease. By monitoring this resonance frequency over time, Brand and Mizaikoff can detect the amount of aromatic hydrocarbons such as benzene present in water.
The researchers plan to run field trials to investigate the use of this new microsensor in aqueous and gaseous environments for rapid on-site screening of multiple pollutants.
“With benzene and other VOCs high on the EPA priority pollutant list, it would be a major advantage to get a rapid reading of VOC concentrations directly in the field,” said Mizaikoff.