Jan 3 2008
The crystal structure of a molecule from a primitive fungus has served as a time machine to show researchers more about the evolution of life from the simple to the complex.
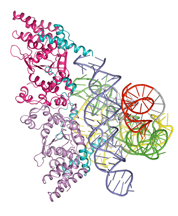 he crystal structure of a molecule from a primitive fungus has served as a time machine to show researchers more about the evolution of life from the simple to the complex.
he crystal structure of a molecule from a primitive fungus has served as a time machine to show researchers more about the evolution of life from the simple to the complex.
By studying the three-dimensional version of the fungus protein bound to an RNA molecule, scientists from Purdue University and the University of Texas at Austin have been able to visualize how life progressed from an early self-replicating molecule that also performed chemical reactions to one in which proteins assumed some of the work.
"Now we can see how RNA progressed to share functions with proteins," said Alan Lambowitz, director of the University of Texas Institute for Cellular and Molecular Biology. "This was a critical missing step."
Results of the study were published in Thursday's (Jan. 3) issue of the journal Nature.
"It's thought that RNA, or a molecule like it, may have been among the first molecules of life, both carrying genetic code that can be transmitted from generation to generation and folding into structures so these molecules could work inside cells," said Purdue structural biologist Barbara Golden. "At some point, RNA evolved and became capable of making proteins. At that point, proteins started taking over roles that RNA played previously - acting as catalysts and building structures in cells."
In order to show this and learn more about the evolution from RNA to more complex life forms, Lambowitz and Paul Paukstelis, lead author and a research scientist at the Texas institute, needed to be able to see how the fungus' protein worked. That's where Golden's team joined the effort and crystallized the molecule at Purdue's macromolecular crystallization facility.
"Obviously, we can't see the process of moving from RNA to RNA and proteins and then to DNA, without a time machine," Golden said. "But by using this fungus protein, we can see this process occurring in modern life."
Looking at the crystal, the scientists saw two things, Golden said. One was that this protein uses two completely different molecular surfaces to perform its two roles. The second is that the protein seems to perform the same job that RNA performed in other simple organisms.
"The crystal structure provides a snapshot of how, during evolution, protein molecules came to assist RNA molecules in their biological functions and ultimately assumed roles previously played by RNA," Golden said.
Before the crystallization, Lambowitz, Paukstelis and their research team at The University of Texas at Austin were involved in a long-term project to study the function of the basic cellular workhorse protein and other evolutionary fossils from the fungus. In earlier work, the scientists studied a different protein that showed how biochemical processes could progress from a world with RNA and protein to DNA.
The protein, as found in the fungus, had adapted to take over some of the RNA molecule's chemical reaction jobs inside cells. The protein stabilizes the RNA molecule - called an intron - so that the RNA can cut out non-functional genetic material and splice together the ends of a functional gene, Paukstelis said.
"The RNA molecule in our study is capable of performing a specific chemical reaction on itself, but it requires a protein for this reaction to take place efficiently," he said.
This basic scientific information eventually could lead to clinical applications.
"This work has potential applications in the development of antifungal drugs to battle potentially deadly pathogens; that's one of the next steps," Lambowitz said. "Another is to produce more detailed structures so that we can understand the ancient chemical reactions."
Golden and Lambowitz are senior authors of the report. Golden is a member of the Markey Center for Structural Biology and Purdue Cancer Center. The Markey Center will be housed in the Hockmeyer Hall of Structural Biology when it's completed on the West Lafayette campus.
Other researchers involved in this study along with Paukstelis were Jui-Hui Chen, a Purdue biochemistry doctoral student, and Elaine Chase, a Purdue biochemistry research technician.