Mar 20 2008
Transport processes in the cells of our body resemble the transport of goods on the roads. Molecular motors, which are special protein molecules, act as trucks. They carry the cellular cargo on piggy-back and transport it along microtubules, which are the roads of the cell.
However, the molecular transporters are a billion times smaller than trucks and can only move as far as the beginning or end of the microtubule, depending on their type. They have to fight their way through a crowd that is more like a busy pedestrian area than a motorway, and also have to compete with motors that want to move in the opposite direction, as scientists at the Max Planck Institute of Colloids and Interfaces in Potsdam have now discovered in a computer simulation.
Several motors are always involved in the tug-of-war over a cargo - for example, some of the kinesin type and some of the dynein type. The kinesin motors move to the end of the microtubule that biologists call the positive end, while the dynein motors move to the minus end. The findings of the Potsdam-based scientists show that the stronger motor team determines the direction in which the cargo is moved. It involves a tug-of-war where opposing motors break off from the microtubule. It was previously assumed that there was a system of coordination that allowed for only one team of motors; it was believed this alternated between one team and the other.
"The tug-of-war is the simplest imaginable mechanism," says Melanie Mueller, one of the scientists involved in the project. "But it is possible, if you consider the properties of the individual motors measured experimentally. They produce a strong non-linear reaction when they are pulled." A motor from the losing team is subject to a strong force and is quickly removed from the microtubule. The remaining motors must then take the force of the winning team alone and are also removed even more quickly. In a domino effect, the losing motors concede and are removed from the microtubule until no others remain. The winning team is then able to transport the cargo quickly, unopposed. "However, the cell does not leave it to chance to ensure that the cargo arrives at its destination. Regulatory proteins probably intervene," says Melanie Mueller.
Researchers into the transport of fat particles in Drosophila embryos examined whether her model applied in reality. It is actually explained by experimental observations that took place previously on the transport mechanism. A cargo in a microtubule does not move directly from one end to the other. It is constantly pulled back in the opposite direction. The losing motors can, however, occasionally remove the winning ones from the microtubule as heat sometimes blows the winning motors away. The cargo particles therefore move in both directions.
"This bi-directional transport process is very flexible," explains Melanie Mueller. It can change direction if the cargo passes its destination or change the speed of the transport. The tug-of-war mechanism, where the winning team pulls the opposing motor team as well as the cargo through the cell, also solves another logistical problem in the cell. It always carries the motors to the end of the microtubule from which they are able to move, preventing an accumulation of motors of one kind at their respective destination.
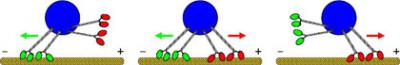 The competition between molecular motors: A blue cargo is transported by two teams of molecular motors moving along the yellow microtubule. The red team of motors is pulling to the right towards the positive end, while the green team is pulling to the left towards the minus end. When both teams pull (in the center), they cancel each other out so the cargo hardly moves forwards. As soon as one team gains the upper hand, it moves quickly as the opposing motors are removed from the microtubule. Credit: Melanie Mueller / MPI of Colloids and Interfaces
The competition between molecular motors: A blue cargo is transported by two teams of molecular motors moving along the yellow microtubule. The red team of motors is pulling to the right towards the positive end, while the green team is pulling to the left towards the minus end. When both teams pull (in the center), they cancel each other out so the cargo hardly moves forwards. As soon as one team gains the upper hand, it moves quickly as the opposing motors are removed from the microtubule. Credit: Melanie Mueller / MPI of Colloids and Interfaces
"Despite the simple mechanism, a cargo particle transported by two motor teams reveals very complex motility behavior," said Melanie Mueller. There are seven different types of motility behavior. These are various combinations of movements to the positive and minus end as well as pauses to which the cargo particles can be subjected. The probability of movement in a certain direction or stopping, and the time lapses between the changes of direction, depend heavily on the properties and the number of the motors involved. The cell uses these to direct the cargo transport. If a team of motors is pulled harder or faster, the cargo moves in the minus instead of the positive direction or stops.
"The simple and efficient tug-of-war mechanism could be used for the transport in micro-laboratories on chips," relates Melanie Mueller. In the same way as with the biological model, teams of motors can transport certain molecules to specific reaction locations on the chip and then also bring back the reaction product. "Our quantitative tug-of-war theory allows motor properties to be optimized for this purpose," according to Mueller.
http://www.mpg.de/