Oct 10 2008
The race for the best "gecko foot" dry adhesive got a new competitor this week with a stronger and more practical material reported in the journal Science by a team of researchers from four U.S. institutions.
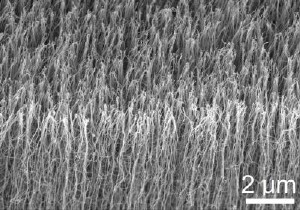 Typical side view of the inverted vertically-aligned multi-walled carbon nanotube film without top entangled segments before adhesion measurements. Credit: Image courtesy of Liangti Qu
Typical side view of the inverted vertically-aligned multi-walled carbon nanotube film without top entangled segments before adhesion measurements. Credit: Image courtesy of Liangti Qu
Scientists have long been interested in the ability of gecko lizards to scurry up walls and cling to ceilings by their toes. The creatures owe this amazing ability to microscopic branched elastic hairs in their toes that take advantage of atomic-scale attractive forces to grip surfaces and support surprisingly heavy loads. Several research groups have attempted to mimic those hairs with structures made of polymers or carbon nanotubes.
In a paper to be published in the October 10 issue of Science, researchers from the University of Dayton, the Georgia Institute of Technology, the Air Force Research Laboratory and the University of Akron describe an improved carbon nanotube-based material that for the first time creates directionally-varied (anisotropic) adhesive force. With a gripping ability nearly three times the previous record - and ten times better than a real gecko at resisting perpendicular shear forces - the new carbon nanotube array could give artificial gecko feet the ability to tightly grip vertical surfaces while being easily lifted off when desired.
Beyond the ability to walk on walls, the material could have many technological applications, including connecting electronic devices and substituting for conventional adhesives in the dry vacuum of space. The research has been sponsored by the National Science Foundation and the U.S. Air Force Research Laboratory at Wright-Patterson Air Force Base near Dayton, Ohio.
"The resistance to shear force keeps the nanotube adhesive attached very strongly to the vertical surface, but you can still remove it from the surface by pulling away from the surface in a normal direction," explained Liming Dai, the Wright Brothers Institute Endowed Chair in the School of Engineering at the University of Dayton. "This directional difference in the adhesion force is a significant improvement that could help make this material useful as a transient adhesive."
The key to the new material is the use of rationally-designed multi-walled carbon nanotubes formed into arrays with "curly entangled tops," said Zhong Lin Wang, a Regents' Professor in the Georgia Tech School of Materials Science and Engineering. The tops, which Wang compared to spaghetti or a jungle of vines, mimic the hierarchical structure of real gecko feet, which include branching hairs of different diameters.
When pressed onto a vertical surface, the tangled portion of the nanotubes becomes aligned in contact with the surface. That dramatically increases the amount of contact between the nanotubes and the surface, maximizing the van der Waals forces that occur at the atomic scale. When lifted off the surface in a direction parallel to the main body of the nanotubes, only the tips remain in contact, minimizing the attraction forces, Wang explained.
"The contact surface area matters a lot," he noted. "When you have line contact along, you have van der Waals forces acting along the entire length of the nanotubes, but when you have a point contact, the van der Waals forces act only at the tip of the nanotubes. That allows us to truly mimic what the gecko does naturally."
In tests done on a variety of surfaces - including glass, a polymer sheet, Teflon and even rough sandpaper - the researchers measured adhesive forces of up 100 Newtons per square centimeter in the shear direction. In the normal direction, the adhesive forces were 10 Newtons per square centimeter - about the same as a real gecko.
The resistance to shear increased with the length of the nanotubes, while the resistance to normal force was independent of tube length.
Though the material might seem most appropriate for use by Spider-Man, the real applications may be less glamorous. Because carbon nanotubes conduct heat and electrical current, the dry adhesive arrays could be used to connect electronic devices.
"Thermal management is a real problem today in electronics, and if you could use a nanotube dry adhesive, you could simply apply the devices and allow van der Waals forces to hold them together," Wang noted. "That would eliminate the heat required for soldering."
Another application might be for adhesives that work long-term in space. "In space, there is a vacuum and traditional kinds of adhesives dry out," Dai noted. "But nanotube dry adhesives would not be bothered by the space environment."
In addition those already mentioned, the research team also included Liangti Qu from the University of Dayton, Morley Stone from the Air Force Research Laboratory, and Zhenhai Xia from the University of Akron.
Qu, a research assistant in the laboratory of Liming Dai, grew the nanotube arrays with a low-pressure chemical vapor deposition process on a silicon wafer. During the pyrolytic growth of the vertically-aligned multi-walled nanotubes, the initial segments grew in random directions and formed a top layer of coiled and entangled nanotubes. This layer helped to increase the nanotube area available for contacting a surface.
Qu noted that sample purity was another key factor in ensuring strong adhesion for the carbon nanotube dry adhesive.
For the future, the researchers hope to learn more about the surface interactions so they can further increase the adhesive force. They also want to study the long-term durability of the adhesive, which in a small number of tests became stronger with each attachment.
And they may also determine how much adhesive might be necessary to support a human wearing tights and red mask.
"Because the surfaces may not be uniform, the adhesive force produced by a larger patch may not increase linearly with the size," Dai said. "There is much we still need to learn about the contact between nanotubes and different surfaces."