Nov 23 2008
If you look carefully at a football, you will notice that its surface is composed of hexagons and pentagons. Hexagons lie side by side while any pentagon is surrounded by five hexagons. How many corners and edges are there? Football players do not have to know that as long as they do pass and shots right; an architect or structural physicist, however, could readily give the answer.
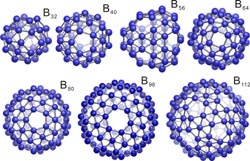 Schematic structures of several boron S-fullerenes.
Schematic structures of several boron S-fullerenes.
For decades, scientists have tried to build molecular structures as symmetric, stable and useful as the football's spheric frame. A rapid communication published online in the Nov. 3 issue of Physical Review B reported a possible recipe for building a variety of nanoscale "footballs" with boron, an element just next to carbon in the periodic table, as proposed by researchers from the College of Physical Sciences, Graduate University of the Chinese Academy of Sciences.
According to Prof. SU Gang, principal investigator of the team, it is well-known that carbon can form nanostructures like fullerenes, nanotubes and graphenes. These structures are endowed with amazing physical and chemical properties: heat resistance, superconductivity, lubrication, even potential medicinal use. Fullerene, for example, is the name of a beautiful carbon-cage molecule found in 1985 by UK and US scientists, who won the Nobel prize in chemistry for the discovery in 1996. Composed entirely of carbon atoms, it may take the form of a hollow sphere, ellipsoid, tube or plane. People named spherical fullerenes "buckyballs" after the noted architectural modeler Buckminster Fuller.
"So boron is just one atomic number from carbon, and they show many structural analogies. Thanks to this proximity, we believe it can also be grown into similar structures," Su noted.
Among the many scientists who have made remarkable research attempts in this regard, there is 1976 Nobel laureate Prof. William N. Lipscomb, who predicted the possibility of a molecule with 32 boron atoms and an icosahedral structure resembling a buckyball shape. In 2004, experts with Yale University synthesized the first pure boron single-wall nanotube in the world. By April 2007, Prof. Yakobson and coworkers at Rice University had envisioned the existence and stability of another buckyball, B80, a hollow spheric cage consisting of 60 boron atoms at corner and an additional one in the center of each hexagon to increase the stability. Recent theoretical studies conducted respectively at Yale and Tsinghua brought about the prediction of new boron sheets and nanotubes.
Based on previous studies, Su's group was devoted to revealing a general scheme for building boron fullerenes. They finally worked out a formula that can derive a large family of new-type fullerenes with outstanding stability. According to the paper, the formula takes the form of "B 32+8k", where "k" can be zero or any counting number. That is to say, theoretically, scientists can develop an unlimited number of structures like B32, B40¡ and so on and so forth, well including the one envisioned by Yakobson. The newly predicted boron sheets, researchers analyzed, could be regarded as an extreme case of the scheme where "k" goes infinite. They call the series S-boron fullerenes, since these buckyballs contain basic building blocks that look like snowdrop motifs.
The researchers then studied the stabilities of these boron fullerenes. With intense ab initio calculations, they proposed an electron counting rule as well as an isolated hollow rule to readily explain high stability and electronic bonding property of the novel structures. Analyses showed that the electronic bonding property was also applicable to a number of newly predicted sheets and nanotubes.
The study by Prof. Su and his colleagues, experts believe, has set up a general framework for the construction and property study of novel boron nanostructures. Meanwhile, it might shed light on the possibility of similar structures built with elements other than carbon and boron.
Now, have you come up with the exact number of corners and edges of a football? Anyway, it has 60 corners and 90 edges.