Mar 16 2009
Materials containing bimetallic nanoparticles are attractive in vast technological fields because of their unique catalytic, electronic, and magnetic properties. One of the most promising of the bunch is made from palladium and gold, an alloy that could be used in a wide variety of catalytic activities including the water-gas shift reaction and the oxidation of carbon dioxide - both important steps in alternative energy applications like fuel cells.
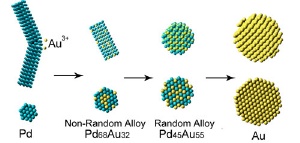 Schematic side view (top) and cross section (bottom) of as-made Pd, Pd68Au32, Pd45Au55, and Au nanomaterial during the galvanic replacement reaction.
Schematic side view (top) and cross section (bottom) of as-made Pd, Pd68Au32, Pd45Au55, and Au nanomaterial during the galvanic replacement reaction.
This alloy has the highest catalytic activity when its structure is "non-random," with a core made predominantly of gold nanoparticles and a shell made mostly of palladium. However, creating the material with traditional chemistry techniques is tricky and difficult to control. Furthermore, establishing a non-random structure of atoms within the nanoparticles requires the use of advanced characterization methods. A team of researchers from Brookhaven, Yeshiva University, and the University of Delaware has demonstrated a new, highly efficient way to synthesize the catalyst and others like it.
"Because a material's performance depends upon its structure, it's important to know how to synthesize its various forms," said Weiqiang Han, a researcher at Brookhaven's Center for Functional Nanomaterials (CFN). "Many techniques have been used to create palladium-gold alloy, but the synthesis of its non-random form with well-controlled atomic distribution is difficult with wet chemistry techniques."
As an alternative method, the researchers used galvanic replacement – the principle behind the technology of batteries. The driving force for galvanic replacement is the electrical potential difference between two metals, with one metal acting principally as the cathode (which gains electrons in the process) and the other metal as the anode (which loses electrons). To create the alloy, the researchers set up a galvanic replacement reaction between palladium nanowires just about 2.5 nanometers thick – one of the thinnest wires reported in the scientific community – and a solution of gold chloride in toluene. They then "watched" the reaction take place through multiple techniques, including electron microscopy and UV-vis absorption at the CFN, x-ray diffraction at NSLS beamline X7B, and extended x-ray absorption fine structure (EXAFS) at NSLS beamline X18B.
"EXAFS is particularly suitable for such investigation since it is extremely sensitive to the structure, geometry, and identity of nearest neighbors in small – less than 3 nm in size – particles," said Anatoly Frenkel, a researcher at Yeshiva University.
In the early stages of the reaction, the researchers found that the resulting alloy was, indeed, non-random, with a gold-rich core and palladium-rich shell. The subsequent addition of the compound alkylamine stabilized palladium to the surface and kept the non-random form intact. However, if the reaction was allowed to continue, a random alloy was formed, with uniformly mixed gold and palladium atoms inside the particles.
Their results were published in the January 23, 2008 edition of the Journal of the American Chemical Society.
"In addition to using this material for several important catalysis applications, the galvanic technique we demonstrated also could be used for the large-scale preparation of other improved non-random alloys," said CFN researcher Xiaowei Teng.
For future studies, the group wants to design different types of non-random catalysts to determine whether similar behavior persists.
Other authors include Ping Liu, Wen Wen, Jonathan Hanson, and Jose Rodriguez , all of Brookhaven; Qi Wang from Yeshiva University; and Nebojsa Marinkovic, from the University of Delaware.
Funding for this study was provided by the U.S. Department of Energy (DOE) and Brookhaven's Laboratory Directed Research and Development Fund. Beamline X18B is supported by the NSLS and the Synchrotron Catalysis Consortium, through DOE's Office of Basic Energy Sciences.