May 3 2009
Yale researchers describe a breakthrough in safe and effective administration of potential antiviral drugs - small interfering RNA (siRNA) molecules that silence genes - the first step in development of a new kind of treatment for sexually transmitted diseases (STDs). The work is reported May 4 as an advance online publication of Nature Materials.
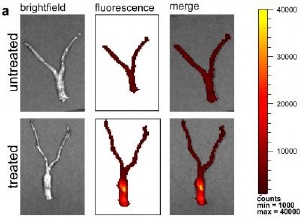 Distribution of nanoparticles seen by fluorescence throughout mouse reproductive tract. Credit: Woodrow/Yale
Distribution of nanoparticles seen by fluorescence throughout mouse reproductive tract. Credit: Woodrow/Yale
"RNA interference is a promising approach for prevention and treatment of human disease," said lead author Kim Woodrow, Yale postdoctoral fellow in Yale's School of Engineering & Applied Science. "We wanted to develop a new strategy of delivering siRNAs with a FDA-approved material."
As their name suggests, siRNAs interfere and knock out the function of genes in higher organism as well as in microbes that may cause STDs. The researchers designed siRNAs to target a gene expressed widely in the lining of the female mouse reproductive tract, in this proof-of-principle work.
Using densely-loaded nanoparticles made of a biodegradable polymer known as PLGA, the researchers created a stable "time release" vehicle for delivery of siRNAs to sensitive mucosal tissue like that of the female reproductive system.
They found that the particles, loaded with the drug agent, moved effectively in two important ways, penetrating to reach cells below the surface of the mucosa and distributing throughout the vaginal, cervical, and uterine regions. Furthermore, the siRNAs stayed in the tissues for at least a week and knockdown of gene activity lasted up to 14 days.
While past work has focused on delivery of siRNAs with liposomes, bubble-like carriers made of phospholipids similar to those found in cell membranes, liposomes are potentially more toxic to the mucosal tissues and are unable to provide sustained release. In the current work, the researchers demonstrated that PLGA nanoparticles were safer than the best current lipid vehicles.
Gene interference therapy is moving rapidly from basic research to application. The PLGA packaging these researchers chose is already approved as safe and non-toxic by the FDA, speeding the path to clinical trials for infectious agents such as HPV and HIV.
"Before human clinical testing can begin, our next step in research will be to test this approach directly in disease models – for example in the HIV model mice that have an immune system genetically identical to humans," said senior author W. Mark Saltzman, the Goizueta Foundation Professor of Biomedical Engineering & Chemical Engineering.
This approach holds promise for global health and the ability of people to self–apply antimicrobial treatments. Woodrow said, "It is safe and effective and much easier than getting an injection of vaccine."