May 15 2009
Of the 92 naturally occurring elements, add another to the list of those that are superconductors. James S. Schilling, Ph.D., professor of physics in Arts + Sciences at Washington University in St. Louis, and Mathew Debessai, Ph.D., - his doctoral student at the time - discovered that europium becomes superconducting at 1.8 K (-456 °F) and 80 GPa (790,000 atmospheres) of pressure, making it the 53rd known elemental superconductor and the 23rd at high pressure.
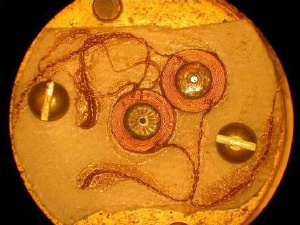 Inside of the diamond cell: In the middle is the coil system around the diamond anvil, which picks up the shielding signal from the superconducting sample.
Inside of the diamond cell: In the middle is the coil system around the diamond anvil, which picks up the shielding signal from the superconducting sample.
Debessai, who received his doctorate in physics at Washington University's Commencement May 15, 2009, is now a postdoctoral research associate at Washington State University.
"It has been seven years since someone discovered a new elemental superconductor," Schilling said. "It gets harder and harder because there are fewer elements left in the periodic table."
This discovery adds data to help improve scientists' theoretical understanding of superconductivity, which could lead to the design of room-temperature superconductors that could be used for efficient energy transport and storage.
The results are published in the May 15, 2009, issue of Physical Review Letters in an article titled "Pressure-induced Superconducting State of Europium Metal at Low Temperatures."
Schilling's research is supported by a four-year $500,000 grant from the National Science Foundation, Division of Materials Research.
Europium belongs to a group of elements called the rare earth elements. These elements are magnetic; therefore, they are not superconductors.
"Superconductivity and magnetism hate each other. To get superconductivity, you have to kill the magnetism," Schilling explained.
Of the rare earths, europium is most likely to lose its magnetism under high pressures due to its electronic structure. In an elemental solid almost all rare earths are trivalent, which means that each atom releases three electrons to conduct electricity.
"However, when europium atoms condense to form a solid, only two electrons per atom are released and europium remains magnetic. Applying sufficient pressure squeezes a third electron out and europium metal becomes trivalent. Trivalent europium is nonmagnetic, thus opening the possibility for it to become superconducting under the right conditions," Schilling said.
Schilling uses a diamond anvil cell to generate such high pressures on a sample. A circular metal gasket separates two opposing 0.17-carat diamond anvils with faces (culets) 0.18 mm in diameter. The sample is placed in a small hole in the gasket, flanked by the faces of the diamond anvils.
Pressure is applied to the sample space by inflating a doughnut-like bellow with helium gas. Much like a woman in stilettos exerts more pressure on the ground than an elephant does because the woman's force is spread over a smaller area, a small amount of helium gas pressure (60 atmospheres) creates a large force (1.5 tons) on the tiny sample space, thus generating extremely high pressures on the sample.
Unique electrical, magnetic properties
Superconducting materials have unique electrical and magnetic properties. They have no electrical resistance, so current will flow through them forever, and they are diamagnetic, meaning that a magnet held above them will levitate.
These properties can be exploited to create powerful magnets for medical imaging, make power lines that transport electricity efficiently or make efficient power generators.
However, there are no known materials that are superconductors at room temperature and pressure. All known superconducting materials have to be cooled to extreme temperatures and/or compressed at high pressure.
"At ambient pressure, the highest temperature at which a material becomes superconducting is 134 K (-218 °F). This material is complex because it is a mixture of five different elements. We do not understand why it is such a good superconductor," Schilling said.
Scientists do not have enough theoretical understanding to be able to design a combination of elements that will be superconductors at room temperature and pressure. Schilling's result provides more data to help refine current theoretical models of superconductivity.
"Theoretically, the elemental solids are relatively easy to understand because they only contain one kind of atom," Schilling said. "By applying pressure, however, we can bring the elemental solids into new regimes, where theory has difficulty understanding things.
"When we understand the element's behavior in these new regimes, we might be able to duplicate it by combining the elements into different compounds that superconduct at higher temperatures."
Schilling will present his findings at the 22nd biennial International Conference on High Pressure Science and Technology in July 2009 in Tokyo, Japan.