Oct 15 2010
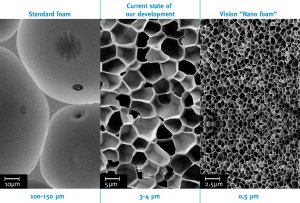
Bayer MaterialScience is working on the development of polyurethane nanofoams that could lead to a quantum leap in thermal insulation performance several years down the road. The company is focusing its efforts on microemulsions, which react under supercritical conditions (Principle of Supercritical Microemulsion Expansion, POSME) to form polyurethane rigid foams. The aim is to produce rigid foams with pore sizes of less than 150 nanometers in diameter.
"Nanofoams of this kind achieve twice the thermal insulation performance of today's polyurethane foams, meaning that they could, for example, significantly reduce the energy consumption of refrigeration appliances and, in turn, make a major contribution to reducing CO2 emissions. Furthermore, the walls of these appliances could be of thinner design, resulting in more storage space for refrigerated goods," explained Dr. Stefan Lindner, a polyurethane rigid foam specialist at Bayer MaterialScience.
 Bayer MaterialScience is working on the development of polyurethane nanofoams that could lead to a quantum leap in thermal insulation performance several years down the road. The company is focusing its efforts on microemulsions, which react under supercritical conditions (Principle of Supercritical Microemulsion Expansion, POSME) to form polyurethane rigid foams. The aim is to produce rigid foams with pore sizes of less than 150 nanometers in diameter. Nanofoams of this kind achieve twice the thermal insulation performance of today's polyurethane foams, meaning that they could, for example, significantly reduce the energy consumption of refrigeration appliances and, in turn, make a major contribution to reducing CO2 emissions.
Bayer MaterialScience is working on the development of polyurethane nanofoams that could lead to a quantum leap in thermal insulation performance several years down the road. The company is focusing its efforts on microemulsions, which react under supercritical conditions (Principle of Supercritical Microemulsion Expansion, POSME) to form polyurethane rigid foams. The aim is to produce rigid foams with pore sizes of less than 150 nanometers in diameter. Nanofoams of this kind achieve twice the thermal insulation performance of today's polyurethane foams, meaning that they could, for example, significantly reduce the energy consumption of refrigeration appliances and, in turn, make a major contribution to reducing CO2 emissions.
The company is partnering on this research project with Prof. Reinhard Strey from the University of Cologne's Institute of Physical Chemistry, who has applied for a patent on the POSME process. As part of the collaboration, his working group is engaged in optimizing the characteristics of the microemulsions.
The thermal insulation performance of a polyurethane rigid foam depends chiefly on the size of the foam pores. The smaller the diameter, the lower the thermal conductivity and the better the insulating effect. Today's polyurethane rigid foams typically have pore sizes of roughly 150 micrometers, which exceeds the pore size of nanofoams planned for the future by a factor of approximately 1,000.
To synthesize a nanofoam using the POSME method, carbon dioxide (CO2) and the liquid polyurethane raw materials (polyol and isocyanate) are mixed with the help of special surfactants at a pressure of 200 bar to form a microemulsion consisting of nanometer-sized droplets filled with CO2 and encapsulated in surfactants. The pressure is then reduced, causing the CO2 to expand and the droplets to become bubbles still in the nanometer range. At the same time, the polyurethane raw materials react to form a 3D polymer network that is a rigid polyurethane foam.
"One of the trickiest challenges we face is to optimally coordinate the reaction of the polyurethane raw materials with the expansion of the CO2 bubbles by carefully fine-tuning the processing parameters so that nanopores of the targeted diameter result," explained Mr. Lindner. "It's no easy task," added Dr. Wolfgang Friederichs, head of Global Product Research at Bayer MaterialScience. "It is likely to take several more years before these challenges are overcome."