A study describing the synthesis of zinc stannate (ZTO) nanostructures through hydrothermia has been published in the March 2011 issue of the journal Science and Technology of Advanced Materials.
It also discusses the physical characteristics and applications of various ZTO nanostructures in solar cells, gas sensors, and photocatalysts. It has been written by Sunandan Baruah and Joydeep Dutta, the Asian Institute of Technology, Klong Luang, Thailand.
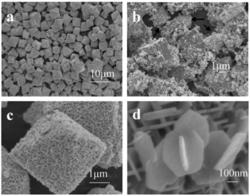 AIT's ZIO Nanostructures
AIT's ZIO Nanostructures
ZTO nanostructures can be fabricated through various ways such as water evaporation, high-temperature calcination, mechanical grinding, sol-gel synthesis, hydrothermal response, and ion-exchange response. Different ratios of ZTO oxides and impurities are derived by using differing techniques, resulting in optional crystal formations. The authors study the features of the hydrothermal technique to synthesize ZTO, such as purity of the zinc orthostannate Zn2SnO4 and the ‘cubic spinel’ crystal formation. This is a simple method because crystals develop at low temperatures in water.
ZTO nanostructures are formed with an aqueous mixture of zinc nitrate or chloride, and stannic chloride, reduced at 200 to 250°C in sodium or ammonium hydroxide under high pressure. The study also describes phase composition or amounts of the oxides created.
ZTO has a bandgap of about 3.6eV, but this energy depends on the synthesis that may result in quantum imprisonment due to the nano-size of the structures. Keeping the photoelectrochemical characteristics of ZTO under control could impact use of other complicated oxides.
A ZTO can degrade hazardous pesticides from ground water, while the porous nanostructures enable gas sensing with enhanced surface to volume ratios. ZTO can also be applied in dye-sensitized solar cells.
Source: http://www.ait.ac.th/