A team comprising global researchers has found a technique to create graphene belts known as nanoribbons. Single-walled carbon nanotubes were un-zipped with the use of hydrogen. This also helped develop graphane nanoribbons, a modified version of the already existing graphene.
The discovery of graphene, a plain, thin carbon flake around one atom thick by Andrei Geim and Konstantin Novoselov awarded them the Nobel prize in physics in 2010. Graphene has unique properties such as it conducts electricity as efficiently as copper. It conducts heat better than the other known materials.
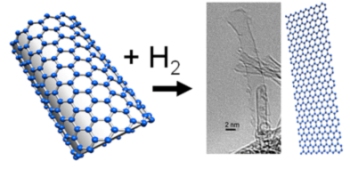 UmU’s Reaction of single-walled carbon nanotubes (SWNTs) with hydrogen gas
UmU’s Reaction of single-walled carbon nanotubes (SWNTs) with hydrogen gas
It is possible to obtain variations in the properties of graphene by making graphene as belts with varying widths known as nanoribbons. They are produced by subjecting carbon nanotubes to oxygen treatment so that they unzip into nanoribbons. However, this procedure leaves oxygen atoms on nanoribbon edges, which is undesirable.
Researches have shown that reactions with molecular hydrogen could unzip single-walled carbon nanotubes. These nanoribbons have hydrogen atoms on the edges that could facilitate some applications. According to Alexandr Talyzin, physicist at Umeå University in Sweden, he has been researching for more than ten years on how hydrogen reacts with fullerenes, or football-shaped carbon molecules and this discovery is an extension of that work.
Nanotubes are sealed with semi-spherical cups that are fullerene molecules divided into two. In an earlier research, the team had discovered that strong hydrogenation could destroy fullerene molecules, yet they tried to unzip the nanotube end cups with hydrogenation and were surprised at the results. Some nanotubes were subjected to unzipping resulting in graphene nanoribbons after hydrogen treatment over long periods. When the nanotube is unzipped with hydrogen bound to the side walls, this results in synthesis of hydrogenated graphene known as graphane.
Until now scientists had tried to synthesize graphane by hydrogen reaction with graphene. This method is not easy when the graphene has been supported on a substrate and one side only can be used for the reaction. Hydrogen reacts easily with curved surfaces of carbon nanotubes.