A team of researchers from the Tsinghua University and Rice University has demonstrated that a combination of carbon nanotube forests and a sulfide-based electrolyte can be used to fabricate high-efficient dye-sensitized solar cells (DSC) at a cost lower than that of conventional silicon-based solar cells.
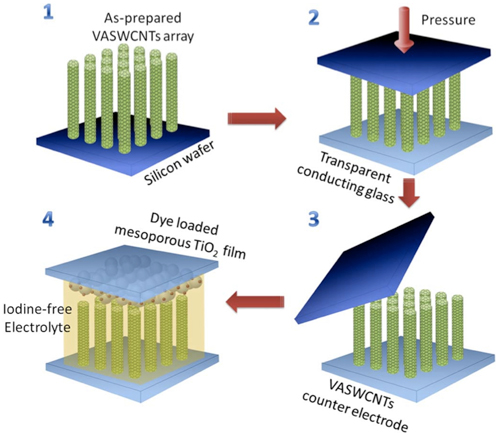 Arrays of vertically aligned single-walled carbon nanotubes (VASWCNTs) grown at Rice University are key to making better and cheaper dye-sensitized solar cells, an alternative to more expensive silicon solar cells. The arrays are transferred to conducting glass, topped with a second electrode of titanium oxide and surrounded by iodine-free electrolyte developed at Tsinghua University. (Lou Lab/Rice University)
Arrays of vertically aligned single-walled carbon nanotubes (VASWCNTs) grown at Rice University are key to making better and cheaper dye-sensitized solar cells, an alternative to more expensive silicon solar cells. The arrays are transferred to conducting glass, topped with a second electrode of titanium oxide and surrounded by iodine-free electrolyte developed at Tsinghua University. (Lou Lab/Rice University)
According to the research team, vertically aligned single-wall carbon nanotube forests developed at the Rice laboratory of Robert Hauge are an efficient replacement to platinum electrodes, a commonly used catalyst in DSC technology. Jun Lou, one of the researchers, informed that these nanotube carpets are also more electroactive than the platinum electrodes. DSCs can be produced more easily than silicon-based solid-state solar cells. However, their efficiency is lower than that of silicon-based solar cells.
In DSCs, photons are absorbed from sunlight by dyes to produce a charge as electrons. A semiconducting titanium oxide layer applied over a current collector captures these electrons before they reach the counter electrode via another current collector. The introduction of iodine-based electrolytes has advanced the DSC production. However, corrosion of metallic current collectors caused by these electrolytes has raised question about the reliability of DSCs, Lou said. Moreover, these electrolytes tend to absorb visible light wavelengths, only fewer photons can be used.
To overcome these issues of iodine-based electrolytes, the Tsinghua researchers developed a sulfide-based electrolyte that is noncorrosive, absorbs little visible light, and is highly compatible with the nanotubes developed by the Rice researchers. Both universities had fabricated working DSCs, with similar results. The power conversion efficiency of these DSCs was 5.25%, a value lower than the DSC record value of 11% achieved using a platinum electrode and iodine electrolytes. However, their conversion efficiency was considerably greater than the value of a control test on the combination of the conventional platinum counter electrode and the new electrolyte.
Lou stated that more research needs to be done because the contact resistance of the carbon nanotube-to-current collector is high and the impact of carbon nanotube structural defects on its catalytic performance is not completely understood.