An international research team has created unique photoluminescent nanoparticles that shine clearly through more than 3 centimeters of biological tissue -- a depth that makes them a promising tool for deep-tissue optical bioimaging.
Though optical imaging is a robust and inexpensive technique commonly used in biomedical applications, current technologies lack the ability to look deep into tissue, the researchers said. This creates a demand for the development of new approaches that provide high-resolution, high-contrast optical bioimaging that doctors and scientists could use to identify tumors or other anomalies deep beneath the skin.
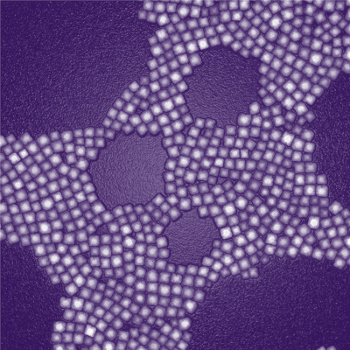 A transmission electron microscopy image of nanoparticles designed for deep-tissue imaging. Each particle consists of a core encased inside a square, calcium-fluoride shell. Photo credit: Zhipeng Li
A transmission electron microscopy image of nanoparticles designed for deep-tissue imaging. Each particle consists of a core encased inside a square, calcium-fluoride shell. Photo credit: Zhipeng Li
The newly created nanoparticles consist of a nanocrystalline core containing thulium, sodium, ytterbium and fluorine, all encased inside a square, calcium-fluoride shell.
The particles are special for several reasons. First, they absorb and emit near-infrared light, with the emitted light having a much shorter wavelength than the absorbed light. This is different from how molecules in biological tissues absorb and emit light, which means that scientists can use the particles to obtain deeper, higher-contrast imaging than traditional fluorescence-based techniques.
Second, the material for the nanoparticles' shell -- calcium fluoride -- is a substance found in bone and tooth mineral. This makes the particles compatible with human biology, reducing the risk of adverse effects. The shell is also found to significantly increase the photoluminescence efficiency.
To emit light, the particles employ a process called near-infrared-to-near-infrared up-conversion, or "NIR-to-NIR." Through this process, the particles absorb pairs of photons and combine these into single, higher-energy photons that are then emitted.
One reason NIR-to-NIR is ideal for optical imaging is that the particles absorb and emit light in the near-infrared region of the electromagnetic spectrum, which helps reduce background interference. This region of the spectrum is known as the "window of optical transparency" for biological tissue, since the biological tissue absorbs and scatters light the least in this range.
The scientists tested the particles in experiments that included imaging them injected in mice, and imaging a capsule full of the particles through a slice of pork more than 3 centimeters thick. In each case, the researchers were able to obtain vibrant, high-contrast images of the particles shining through tissue.
The results of the study appeared online on Aug. 28 in the ACS Nano journal. The international collaboration included researchers from the University at Buffalo and other institutions in the U.S., China, South Korea and Sweden. It was co-led by Paras N. Prasad, a SUNY Distinguished Professor and executive director of UB's Institute for Lasers, Photonics and Biophotonics (ILPB), and Gang Han, an assistant professor at University of Massachusetts Medical School.
"We expect that the unprecendented properties in the core/shell nanocrystals we designed will bridge numermous disconnections between in vitro and in vivo studies, and eventully lead to new discoveries in the fields of biology and medicine," said Han, expressing his excitement about the research findings.
Study co-author Tymish Y. Ohulchanskyy, a deputy director of ILPB, believes the 3-centimeter optical imaging depth is unprecedented for nanoparticles that provide such high-contrast visualization.
"Medical imaging is an emerging area, and optical imaging is an important technique in this area," said Ohulchanskyy. "Developing this new nanoplatform is a real step forward for deeper tissue optical bioimaging."
The paper's first authors were Guanying Chen, research assistant professor at ILPB and scientist at China's Harbin Institute of Technology and Sweden's Royal Institute of Technology and Jie Shen of the University of Massachusetts Medical School. Other institutions that contributed included Roswell Park Cancer Institute, the University of North Carolina at Chapel Hill and Korea University at Seoul.
The next step in the research is to explore ways of targeting the nanoparticles to cancer cells and other biological targets that could be imaged. Chen, Shen and Ohulchanskyy said the hope is for the nanoparticles to become a platform for multimodal bioimaging.