Dec 21 2012
The research group of Professor Petri Pihko at the Department of Chemistry and the NanoScience Center of the University of Jyväskylä has solved two acute problems in chemical catalysis. The research has been funded by the Academy of Finland.
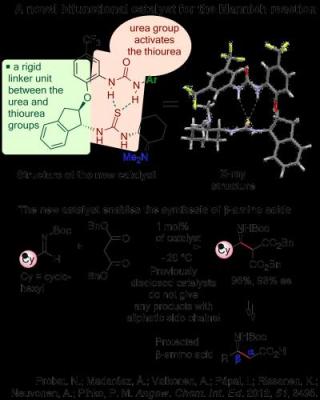 This image shows a novel bifunctional catalyst for the Mannich reaction. Credit: Professor Petri Pihko
This image shows a novel bifunctional catalyst for the Mannich reaction. Credit: Professor Petri Pihko
In the first project, the researchers designed a novel intramolecularly assisted catalyst for the synthesis of beta amino acids. Previously published catalysts work only with aromatic side chains in the imines, but the new catalyst designed at Jyväskylä does not have this limitation. The new method might find uses in the synthesis of beta amino acids, which are important building blocks for chemical biology. For the understanding of the catalytic mechanism and design of the catalyst, the researchers collaborated with the group of Imre Pápai (Hungarian Academy of Sciences, computational studies) and Academy Professor Kari Rissanen (Jyväskylä, X-ray characterisation of catalysts).
In the second project, the researchers identified a completely new mechanism for the amine-catalysed Michael addition reaction between aldehydes and nitroalkenes. The mechanism has been a source of intense discussion within the scientific community, with the groups of Professor Yujiro Hayashi (Tokyo), Professor Donna Blackmond (La Jolla, USA) and Professor Dieter Seebach (ETH, Switzerland) each presenting different possible mechanisms.
The new model proposed by the Pihko and Papai groups includes a new species, a six-membered ring, as the key on-cycle intermediate that is protonated in the rate-determining step. The work is a combination of computational and experimental studies that complement each other in understanding the mechanism and demonstrate how difficult mechanistic puzzles can be solved by joining the forces of both approaches.