Aug 1 2013
University of Oregon chemist David C. Johnson likens his lab's newly published accomplishments to combining two flavors of ice cream — vanilla and chocolate — and churning out thousands of flavors to appeal to any taste bud.
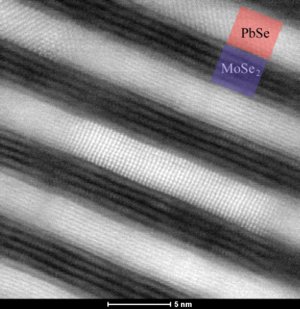 Atoms placed layers as seen with high-resolution STEM-HAADF tomography
Atoms placed layers as seen with high-resolution STEM-HAADF tomography
In reality, though, he is referring to his game-changing approach to synthesize thousands of new compounds with ultra low thermal conductivity and other unusual properties quickly instead of the traditional and time-consuming preparative approach that is limited to just a few thermodynamically stable compounds.
In a paper placed online ahead of print publication by the Journal of the American Chemical Society (JACS), Johnson's eight-member team documented their design of layered elemental precursors that when lightly warmed self assemble into 18 new nano-sized and metastable compounds with predictable nano-architectures and specific crystallographic orientations.
Until recently, the design and synthesis of crystalline materials in solid-state inorganic chemistry has been very limited because creating them has required heating at high temperatures for long periods, with little control of the reaction pathway. Johnson's new approach is a paradigm change.
"We can now make 20,000 compounds where there used to be three," Johnson said. "We've gone from thermodynamic control to kinetic control. In this particular case, we've taken two compounds, and we can take these solids and interweave them — put them together however we want by rearranging the layers. We can make specific structures that have subtle differences in their physical properties."
The key to the success of the new approach, he said, involves controlling the local compositions and diffusion distances of the precursor to guide the formation process so the constituent layers come together predictably.
"We design and calibrate the repeating unit of the precursor such that the relative and absolute amounts of atoms of each element within each region of the precursor corresponds to the number of atoms needed to form complete structural units of each component on annealing," the researchers wrote in their paper. "If too much or too little material is present, we found that the resulting partial layers disrupt the desired ordered structure."
Another challenge also emerges when working at nano sizes; as crystals get smaller the ratio of bulk to surface atoms decreases, leading to changes in their structure. Due to the distributions of sizes resulting from other synthesis techniques, the exact structures of nano-sized crystals have not been determined.
Earlier this year, in the Feb. 11 issue of the journal Angewandte, another Johnson-led team described how his lab's synthesis technique produces precisely defined structures, which permit distortions in structure to be experimentally determined as a function of size. This structural knowledge, he said, is key to predicting how properties change with nanoarchitecture.
Just how the new technology will be utilized isn't clear, said Johnson, who holds the Rosaria P. Haugland Foundation Chair in Pure and Applied Chemistry at the UO. "We're making lab-scale sample films that are only a fraction of a hair's thickness," he said.
Their ultra-low thermal conductivity makes insulation a most-likely early scenario for application, he said, but his lab in the Center for Sustainable Materials Chemistry — a National Science Foundation-funded center involving the UO and Oregon State University — is now looking at how to possibly increase production by way of a cost-effective solution route to create coatings that self-organize into one of these low thermal conductivity solids.
"They are fantastic insulators," said Johnson, who also is a member of the UO's Materials Science Institute. "Most solids like window glass have a thermal conductivity of about 1 watt per meter kelvin. Air, on the other hand, has a thermal conductivity of about .03 watts per meter kelvin. Our materials have a thermal conductivity of about .05-0.10, which means that if the material was at about 500 degrees Celsius, I could touch it. There wouldn't be enough energy transferred per unit time to burn my hand."
"UO researchers are helping to create a more sustainable future by re-engineering the science, manufacturing and business processes related to critical products," said Kimberly Andrews Espy, the UO's vice president for research and innovation and dean of the graduate school. "This approach by Dr. Johnson and his team, which enables the quick synthesis of thousands of new compounds, holds enormous potential for the creation of new materials."
The seven co-authors with Johnson on the JACS paper were: Colby L. Heideman, who earned a doctorate in chemistry at the UO and is at Eastern Oregon University in La Grande; Sara Tepfer, who earned a bachelor's degree in chemistry from the UO; Qiyin Lin, who earned a doctorate in physics at the UO and is at the University of California, Irvine; Raimar Rostek, a doctoral student at Germany's University of Freiburg who completed his master's degree thesis while working in Johnson's lab; Paul Zschack of the Argonne National Laboratory near Chicago; Michael Dale Anderson, who earned his doctorate in chemistry from the UO and was at the National Institute of Standards and Technology (NIST) in Gaithersburg, Md.; and Ian M. Anderson, also of NIST.
The National Science Foundation supported the research primarily through grants DRM-0907049, MRI-09233577 and CHE-1102637. Heideman and Michael Anderson were supported by an NSF IGERT Fellowship grant to the UO (DGE-0549503). Some of the research also utilized the U.S. Department of Energy-supported Advanced Photon Source at the Argonne National Laboratory.