Dec 16 2013
Fuel cells represent an important component of the energy transition, as they supply electrical energy without first having to create heat and steam from fossil fuels. Instead, they create the energy directly from a reaction of hydrogen and oxygen to form water. For that reason, they can create energy more efficiently than coal-fired or gas-fired power plants.
Today’s fuel cells require, however, costly platinum as a catalyst for this reaction, which restricts their more widespread use. A team at the Max Planck Institute for Solid State Research in Stuttgart has been inspired by nature to develop an alternative catalyst. It consists of organic molecules as well as iron or manganese on a metallic substrate. These materials are less costly and more easily available than platinum.
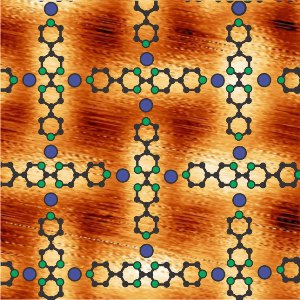 The iron atoms (blue) and the organic molecules (green, black) form a lattice pattern on the gold substrate. (© Grumelli et al., Nat. Comm. 2013)
The iron atoms (blue) and the organic molecules (green, black) form a lattice pattern on the gold substrate. (© Grumelli et al., Nat. Comm. 2013)
Humans and animals obtain energy from the same reaction as fuel cells: they breathe in oxygen and bind hydrogen with it in their cells to form water. In this chemical conversion, energy is released, which the organism uses to live. Therefore, the idea to search in nature for a catalyst as a substitute for expensive platinum is logical. This noble metal drives a specific partial reaction during the energy conversion in a fuel cell: the so-called reduction of oxygen. During this reaction, oxygen picks up either two or four electrons, depending on whether it reacts directly with hydrogen or via an intermediate hydrogen peroxide molecule to form water.
Natural oxygen-reducing enzymes contain metals like iron and manganese, which are easily obtained through nutritional sources. Organic molecules associated with this enzyme hold on tightly to the atoms of these metals, so that the oxygen can dock there and be reduced. Klaus Kern and his staff member Doris Grumelli from the Max Planck Institute for Solid State Research have now evaporated iron and manganese atoms together with organic molecules onto a gold substrate. In doing this, they established that the organic molecules and the metal atoms become ordered in patterns that strongly resemble the functional centres of enzymes. Networks formed in which individual iron or manganese atoms are surrounded by several organic molecules, like the intersecting points in a lattice fence.
In order to be able to test how the catalyst functions in the networks, the researchers had to develop a transport system with which they can move the samples from vacuum into liquids. This is because the new surface structures were formed under a very high vacuum, while the tests took place outside the vacuum chamber in an electrochemical cell. It turned out that the catalytic activity depended of the kind of metallic centre, while, on the other hand, the stability of the structure depended on the type of organic molecules that form the network. Iron atoms led to a two-level reaction via the intermediate hydrogen peroxide molecule, while manganese atoms produced a direct reaction of oxygen to water.
The latter reaction would be interest for fuel cells, as experts expect higher efficiency in converting the chemical energy to electrical energy through the direct reaction. “However, the other variant could also have applications," says Grumelli, “even serving to modulate or interrupt the reaction.” This can play a role in biosensors, for example. In any event, the Group has succeeded in making a new class of nanocatalysts that are cost-effective to manufacture, and whose raw materials are plentiful. Doris Grumelli is already working on a new variant of these kinds of structures: with the help of special organic molecules which each contain a metal atom, and the use of additional metal atoms, she hopes to create a surface structure that simultaneously contains two types of metal atoms. “Such structures could serve as models for biological research,” says the scientist.