May 5 2014
In an electric car you want a battery that has enough juice to get you across town and enough kick to accelerate onto the highway. That is, the amount of energy a battery stores and the rate at which it releases that energy determines the usefulness of the battery.
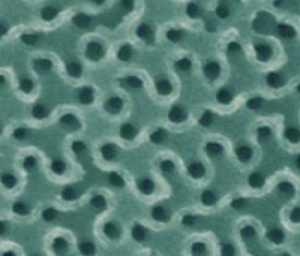 "A scanning electron microscopy image shows the wavy pattern of a gyroidal mesoporous carbon."
"A scanning electron microscopy image shows the wavy pattern of a gyroidal mesoporous carbon."
A porous carbon electrode developed by researchers at the Energy Materials Center at Cornell (emc2) presents a platform to significantly improve a battery's kick and decrease charging times. When a battery operates, positively charged ions move from one side of the battery to the other through a necessary separation layer. The shorter the distance these ions have to travel, the faster a battery can both release and store energy, but thinner batteries also store less energy.
The solution to making a thin battery store a sufficient amount of energy is to extend the thin battery into the third dimension. So-called 3-D batteries are of great commercial interest, and a highly porous, conductive carbon host is an excellent framework that 3-D batteries could be built around.
The best carbon hosts for battery electrodes have pores that are highly ordered and interconnected to provide large surface areas. Mesoscale (about 2 to 50 nanometers wide) pores are expected to be large enough that they can be filled with all of the components of a battery but small enough that ions can quickly move from one side of the filled pore to the other during operation.
Jörg Werner, Tobias Hoheisel, and Uli Wiesner of emc2 report the largest, most interconnected, and most thermally stable pores in a mesoporous carbon made to date using soft templating methods. The large pore size (about 40 nanometers) of the team's mesoporous carbons is ideal for clog-free filling of the pores.
In this work, a carbon precursor was mixed in solution with a custom polymer that served as the soft template. As the solvent evaporated, the polymer and carbon precursor self-assembled into a highly ordered and interconnected structure called a gyroid. The polymer template was removed by decomposition with heat, and the remaining carbon precursor was converted to graphite with further heat treatment, maintaining structural integrity at the highest reported temperatures for soft-templated carbons.
From a commercial standpoint, soft-templating methods enable roll-to-roll fabrication of mesoporous carbons with fewer steps and fewer harsh chemicals than strategies using a hard template such as silica.
The soft template synthetic approach also allowed the size of the pores and thickness of the pore walls to be tuned over many nanometers by varying the size of the polymer and the amount of the carbon precursor. Tuning the dimensions of pores in a battery with a mesoporous carbon host would afford control over the amount of energy stored and the rate of energy release (power output) in a device.
The mesoporous carbons were made as free-standing macroscopic materials with tunable thickness and can be cut into a desired shape for a given battery application. Having established a soft-template synthetic strategy for making ordered mesoporous carbons with large pores, the next milestone is filling the pores with the individual components of a battery.